UDP-galactose (SLC35A2) and UDP-N-acetylglucosamine (SLC35A3) Transporters Form Glycosylation-related Complexes with Mannoside Acetylglucosaminyltransferases (Mgats)
- PMID: 25944901
- PMCID: PMC4505462
- DOI: 10.1074/jbc.M115.636670
UDP-galactose (SLC35A2) and UDP-N-acetylglucosamine (SLC35A3) Transporters Form Glycosylation-related Complexes with Mannoside Acetylglucosaminyltransferases (Mgats)
Abstract
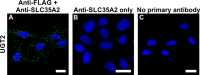
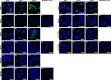
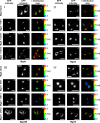
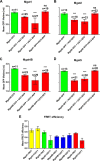
UDP-galactose transporter (UGT; SLC35A2) and UDP-N-acetylglucosamine transporter (NGT; SLC35A3) form heterologous complexes in the Golgi membrane. NGT occurs in close proximity to mannosyl (α-1,6-)-glycoprotein β-1,6-N-acetylglucosaminyltransferase (Mgat5). In this study we analyzed whether NGT and both splice variants of UGT (UGT1 and UGT2) are able to interact with four different mannoside acetylglucosaminyltransferases (Mgat1, Mgat2, Mgat4B, and Mgat5). Using an in situ proximity ligation assay, we found that all examined glycosyltransferases are in the vicinity of these UDP-sugar transporters both at the endogenous level and upon overexpression. This observation was confirmed via the FLIM-FRET approach for both NGT and UGT1 complexes with Mgats. This study reports for the first time close proximity between endogenous nucleotide sugar transporters and glycosyltransferases. We also observed that among all analyzed Mgats, only Mgat4B occurs in close proximity to UGT2, whereas the other three Mgats are more distant from UGT2, and it was only possible to visualize their vicinity using proximity ligation assay. This strongly suggests that the distance between these protein pairs is longer than 10 nm but at the same time shorter than 40 nm. This study adds to the understanding of glycosylation, one of the most important post-translational modifications, which affects the majority of macromolecules. Our research shows that complex formation between nucleotide sugar transporters and glycosyltransferases might be a more common phenomenon than previously thought.
Keywords: Golgi; UDP-N-acetylglucosamine transporter; UDP-galactose transporter; fluorescence resonance energy transfer (FRET); glycosylation; glycosyltransferase; mannoside acetylglucosaminyltransferase; protein complex; proximity ligation assay; sugar transport.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Coates S. W., Gurney T., Jr., Sommers L. W., Yeh M., Hirschberg C. B. (1980) Subcellular localization of sugar nucleotide synthetases. J. Biol. Chem. 255, 9225–9229 - PubMed
-
- Gerardy-Schahn R., Oelmann S., Bakker H. (2001) Nucleotide sugar transporters: Biological and functional aspects. Biochimie 83, 775–782 - PubMed
-
- Caffaro C. E., Hirschberg C. B. (2006) Nucleotide sugar transporters of the Golgi apparatus: from basic science to diseases. Acc. Chem. Res. 39, 805–812 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

