Murine study of portal hypertension associated endothelin-1 hypo-response
- PMID: 25944995
- PMCID: PMC4408454
- DOI: 10.3748/wjg.v21.i16.4817
Murine study of portal hypertension associated endothelin-1 hypo-response
Abstract
Aim: To investigate endothelin-1 hypo-responsive associated with portal hypertension in order to improve patient treatment outcomes.
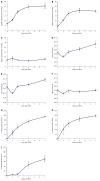
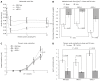
Methods: Wild type, eNOS(-/-) and iNOS(-/-) mice received partial portal vein ligation surgery to induce portal hypertension or sham surgery. Development of portal hypertension was determined by measuring the splenic pulp pressure, abdominal aortic flow and portal systemic shunting. To measure splenic pulp pressure, a microtip pressure transducer was inserted into the spleen pulp. Abdominal aortic flow was measured by placing an ultrasonic Doppler flow probe around the abdominal aorta between the diaphragm and celiac artery. Portal systemic shunting was calculated by injection of fluorescent microspheres in to the splenic vein and determining the percentage accumulation of spheres in liver and pulmonary beds. Endothelin-1 hypo-response was evaluated by measuring the change in abdominal aortic flow in response to endothelin-1 intravenous administration. In addition, thoracic aorta endothelin-1 contraction was measured in 5 mm isolated thoracic aorta rings ex-vivo using an ADI small vessel myograph.
Results: In wild type and iNOS(-/-) mice splenic pulp pressure increased from 7.5 ± 1.1 mmHg and 7.2 ± 1 mmHg to 25.4 ± 3.1 mmHg and 22 ± 4 mmHg respectively. In eNOS(-/-) mice splenic pulp pressure was increased after 1 d (P = NS), after which it decreased and by 7 d was not significantly elevated when compared to 7 d sham operated controls (6.9 ± 0.6 mmHg and 7.3 ± 0.8 mmHg respectively, P = 0.3). Abdominal aortic flow was increased by 80% and 73% in 7 d portal vein ligated wild type and iNOS when compared to shams, whereas there was no significant difference in 7 d portal vein ligated eNOS(-/-) mice when compared to shams. Endothelin-1 induced a rapid reduction in abdominal aortic blood flow in wild type, eNOS(-/-) and iNOS(-/-) sham mice (50% ± 8%, 73% ± 9% and 47% ± 9% respectively). Following portal vein ligation endothelin-1 reduction in blood flow was significantly diminished in each mouse group. Abdominal aortic flow was reduced by 19% ± 9%, 32% ± 10% and 9% ± 9% in wild type, eNOS(-/-) and iNOS(-/-) mice respectively.
Conclusion: Aberrant endothelin-1 response in murine portal hypertension is NOS isoform independent. Moreover, portal hypertension in the portal vein ligation model is independent of ET-1 function.
Keywords: Endothelin-1; Hyper-dynamic circulation; Liver disease; Nitric oxide synthase isoforms; Portal hypertension.
Figures

References
-
- Reichen J. Liver function and pharmacological considerations in pathogenesis and treatment of portal hypertension. Hepatology. 1990;11:1066–1078. - PubMed
-
- Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol. 1983;244:G52–G57. - PubMed
-
- Laleman W, Nevens F. Cirrhotic portal hypertension: current and future medical therapy for primary and secondary prevention of variceal bleeding. Minerva Med. 2006;97:325–345. - PubMed
-
- Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2–5. - PubMed
-
- Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300–1314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

