Ginseng Purified Dry Extract, BST204, Improved Cancer Chemotherapy-Related Fatigue and Toxicity in Mice
- PMID: 25945105
- PMCID: PMC4405287
- DOI: 10.1155/2015/197459
Ginseng Purified Dry Extract, BST204, Improved Cancer Chemotherapy-Related Fatigue and Toxicity in Mice
Abstract
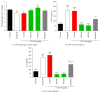
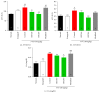
Cancer related fatigue (CRF) is one of the most common side effects of cancer and its treatments. A large proportion of cancer patients experience cancer-related physical and central fatigue so new strategies are needed for treatment and improved survival of these patients. BST204 was prepared by incubating crude ginseng extract with ginsenoside-β-glucosidase. The purpose of the present study was to examine the effects of BST204, mixture of ginsenosides on 5-fluorouracil (5-FU)-induced CRF, the glycogen synthesis, and biochemical parameters in mice. The mice were randomly divided into the following groups: the naïve normal (normal), the HT-29 cell inoculated (xenograft), xenograft and 5-FU treated (control), xenograft + 5-FU + BST204-treated (100 and 200 mg/kg) (BST204), and xenograft + 5-FU + modafinil (13 mg/kg) treated group (modafinil). Running wheel activity and forced swimming test were used for evaluation of CRF. Muscle glycogen, serum inflammatory cytokines, aspartic aminotransferase (AST), alanine aminotransferase (ALT), creatinine (CRE), white blood cell (WBC), neutrophil (NEUT), red blood cell (RBC), and hemoglobin (HGB) were measured. Treatment with BST204 significantly increased the running wheel activity and forced swimming time compared to the control group. Consistent with the behavioral data, BST204 markedly increased muscle glycogen activity and concentrations of WBC, NEUT, RBC, and HGB. Also, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), AST, ALT, and CRE levels in the serum were significantly reduced in the BST204-treated group compared to the control group. This result suggests that BST204 may improve chemotherapy-related fatigue and adverse toxic side effects.
Figures





Similar articles
-
Treatment of chemotherapy-induced cachexia with BST204: a multimodal validation study.Metabolomics. 2021 Mar 18;17(4):36. doi: 10.1007/s11306-021-01781-8. Metabolomics. 2021. PMID: 33738589
-
BST204, a Rg3 and Rh2 Enriched Ginseng Extract, Upregulates Myotube Formation and Mitochondrial Function in TNF-α-Induced Atrophic Myotubes.Am J Chin Med. 2020;48(3):631-650. doi: 10.1142/S0192415X20500329. Epub 2020 Apr 24. Am J Chin Med. 2020. PMID: 32329640
-
Pharmacokinetics and tissue distribution of ginsenoside Rh2 and Rg3 epimers after oral administration of BST204, a purified ginseng dry extract, in rats.Xenobiotica. 2014 Dec;44(12):1099-107. doi: 10.3109/00498254.2014.929192. Epub 2014 Jun 16. Xenobiotica. 2014. PMID: 24933530
-
Evaluation of the in vitro/in vivo drug interaction potential of BST204, a purified dry extract of ginseng, and its four bioactive ginsenosides through cytochrome P450 inhibition/induction and UDP-glucuronosyltransferase inhibition.Food Chem Toxicol. 2014 Jun;68:117-27. doi: 10.1016/j.fct.2014.03.004. Epub 2014 Mar 12. Food Chem Toxicol. 2014. PMID: 24632066
-
Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages.Int Immunopharmacol. 2005 May;5(5):929-36. doi: 10.1016/j.intimp.2005.01.008. Int Immunopharmacol. 2005. PMID: 15778128
Cited by
-
Comprehensive review of mouse models for studying cancer-related fatigue: Methods, findings and future directions (Review).Exp Ther Med. 2025 Jul 21;30(3):178. doi: 10.3892/etm.2025.12928. eCollection 2025 Sep. Exp Ther Med. 2025. PMID: 40746448 Free PMC article. Review.
-
BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function.Int J Environ Res Public Health. 2021 Mar 1;18(5):2367. doi: 10.3390/ijerph18052367. Int J Environ Res Public Health. 2021. PMID: 33804338 Free PMC article.
-
Effects of the purified dry extract of fermented ginseng BST204 on muscle fiber regeneration.Biochem Biophys Rep. 2023 Aug 14;35:101525. doi: 10.1016/j.bbrep.2023.101525. eCollection 2023 Sep. Biochem Biophys Rep. 2023. PMID: 37601455 Free PMC article.
-
Treatment of chemotherapy-induced cachexia with BST204: a multimodal validation study.Metabolomics. 2021 Mar 18;17(4):36. doi: 10.1007/s11306-021-01781-8. Metabolomics. 2021. PMID: 33738589
-
The Effect of Earthing Mat on Stress-Induced Anxiety-like Behavior and Neuroendocrine Changes in the Rat.Biomedicines. 2022 Dec 26;11(1):57. doi: 10.3390/biomedicines11010057. Biomedicines. 2022. PMID: 36672565 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

