Nerve growth factor in human semen: Effect of nerve growth factor on the normozoospermic men during cryopreservation process
- PMID: 25945243
- PMCID: PMC4414996
Nerve growth factor in human semen: Effect of nerve growth factor on the normozoospermic men during cryopreservation process
Abstract
Objectives: Although routinely applied in assisted reproductive technology, human sperm cryopreservation is not a completely successful procedure. Adverse effects of cryopreservation on the fertilization capacity, motility, morphology, and viability of spermatozoa have been proven; cryopreservation has also shown a role in sperm DNA fragmentation and infertility. The post-thaw survival of spermatozoa improved after addition of supplementation of antioxidant molecules to freezing media. Nerve growth factor (NGF) as one of the prosurvival substances has gained great attention in recent years. The aim of this study was the usage of NGF as prosurvival factor after cryopreservation process of human semen samples to assess the motility and viability of sperm, nitric oxide (NO) concentration, and DNA fragmentation in normozoospermic men.
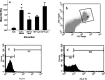
Materials and methods: Semen samples were collected from 25 normozoospermic men and were divided into fresh semen samples as control group, frozen-thawed semen samples without addition of exogenous NGF, and three groups of semen samples cryopreserved with addition of exogenous NGF (0.5, 1, and 5 ng/ml) in freezing medium. Viability was assessed by eosin-negrosin staining technique. Motility was evaluated with inverted microscope. NO concentration and apoptosis content were measured with flow cytometry.
Results: Results showed that exogenous NGF at 0.5 ng/ml could significantly (P-value <0.05) influence viability, motility, nitric oxide, and DNA fragmentation content.
Conclusion: Exogenous NGF as cryoprotectant improved sperm viability and motility, increased intracellular NO concentration, and decreased apoptosis content in normal human spermatozoa.
Keywords: Apoptosis; Cryopreservation; Human sperm; Nerve growth factor; Nitric oxide.
Figures

References
-
- Lassalle B, Testart J. Human zona pellucida recognition associated with removal of sialic acid from human sperm surface. J Reprod Fertil. 1994;101:703–711. - PubMed
-
- Du Plessis SS, Makker K, Desai NR, Agarwal A. The impact of oxidative stress on in vitro fertilization. Expert Rev Obstet Gynecol. 2008;3:539–554.
-
- Du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, Lampiao F. Effects of H2O2 exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42:206–210. - PubMed
LinkOut - more resources
Full Text Sources
Medical
