High-intensity focused ultrasound for the treatment of fibroadenomata (HIFU-F) study
- PMID: 25945250
- PMCID: PMC4419404
- DOI: 10.1186/s40349-015-0027-6
High-intensity focused ultrasound for the treatment of fibroadenomata (HIFU-F) study
Abstract
Background: Breast fibroadenomata (FAD) are the most common benign lesions in women. For palpable lesions, there are currently three standard treatment options: reassurance (with or without follow-up), vacuum-assisted mammotomy (VAM) or surgical excision. High-intensity focused ultrasound (HIFU) ablation has been used in the treatment of FAD. The drawback of HIFU is its prolonged treatment duration. The aim of this trial is to evaluate circumferential HIFU treatment for the effective ablation of FAD with a reduced treatment time.
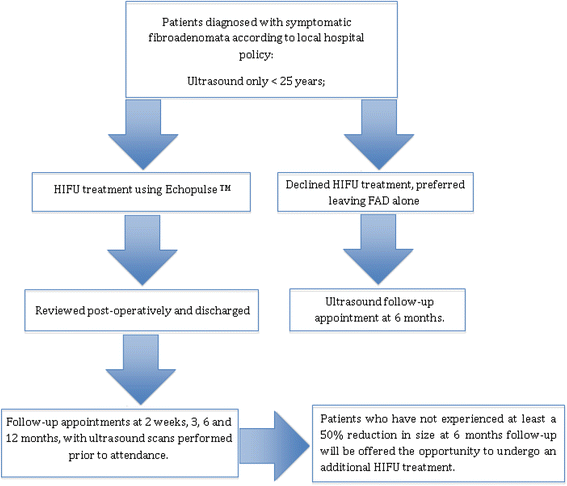
Methods/design: Fifty patients (age ≥18 years) will be recruited with symptomatic FAD, visible on ultrasound (US, grade U2 benign). In patients ≥25 years, cytology or histology will be performed to confirm the diagnosis of a FAD. These patients will receive HIFU treatment using the US-guided Echopulse device (Theraclion Ltd., Malakoff, France) under local anaesthesia. An additional 50 patients will be recruited and contacted 6 months after discharge from the breast clinic. These patients will be offered an US scan to determine the change in size of their FAD. This natural change in size will be compared to the decrease in size after HIFU treatment. Secondary outcome measures include post-treatment complications, patient recorded outcome measures, mean treatment time and cost analysis.
Trial registration: Current Controlled Trials: ISRCTN76622747.
Keywords: Benign breast disease; Fibroadenoma; Non-invasive treatment; Ultrasound-guided high-intensity focused ultrasound (HIFU).
Similar articles
-
Five-year follow-up after a single US-guided high intensity focused ultrasound treatment of breast fibroadenoma.Sci Rep. 2024 Aug 7;14(1):18370. doi: 10.1038/s41598-024-68827-4. Sci Rep. 2024. PMID: 39112604 Free PMC article.
-
A review of ablative techniques in the treatment of breast fibroadenomata.J Ther Ultrasound. 2016 Jan 19;4:1. doi: 10.1186/s40349-016-0045-z. eCollection 2016. J Ther Ultrasound. 2016. PMID: 26788322 Free PMC article. Review.
-
High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial.Int J Hyperthermia. 2016 Dec;32(8):881-888. doi: 10.1080/02656736.2016.1212278. Epub 2016 Sep 7. Int J Hyperthermia. 2016. PMID: 27484113 Clinical Trial.
-
High-intensity focused ultrasound in the treatment of breast fibroadenomata (HIFU-F trial).Int J Hyperthermia. 2018 Nov;34(7):1002-1009. doi: 10.1080/02656736.2017.1373865. Epub 2017 Oct 2. Int J Hyperthermia. 2018. PMID: 28854826
-
High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules - a systematic review.J Ther Ultrasound. 2017 May 17;5:11. doi: 10.1186/s40349-017-0091-1. eCollection 2017. J Ther Ultrasound. 2017. PMID: 28523127 Free PMC article. Review.
Cited by
-
Five-year follow-up after a single US-guided high intensity focused ultrasound treatment of breast fibroadenoma.Sci Rep. 2024 Aug 7;14(1):18370. doi: 10.1038/s41598-024-68827-4. Sci Rep. 2024. PMID: 39112604 Free PMC article.
-
Focused ultrasound radiosensitizes human cancer cells by enhancement of DNA damage.Strahlenther Onkol. 2021 Aug;197(8):730-743. doi: 10.1007/s00066-021-01774-5. Epub 2021 Apr 22. Strahlenther Onkol. 2021. PMID: 33885910 Free PMC article.
-
A review of ablative techniques in the treatment of breast fibroadenomata.J Ther Ultrasound. 2016 Jan 19;4:1. doi: 10.1186/s40349-016-0045-z. eCollection 2016. J Ther Ultrasound. 2016. PMID: 26788322 Free PMC article. Review.
-
From Anatomy to Functional and Molecular Biomarker Imaging and Therapy: Ultrasound Is Safe, Ultrafast, Portable, and Inexpensive.Invest Radiol. 2020 Sep;55(9):559-572. doi: 10.1097/RLI.0000000000000675. Invest Radiol. 2020. PMID: 32776766 Free PMC article. Review.
References
-
- Ozzello L, Gump FE. The management of patients with carcinomas in fibroadenomatous tumors of the breast. Surg Gynecol Obstet. 1985;160(2):99–104. - PubMed
-
- Fibroadenoma of the breast. Atlas of Pathology. 2009. http://www.pathologyatlas.ro/fibroadenoma-breast-pathology.php. Accessed 20–10 2014.
LinkOut - more resources
Full Text Sources
Other Literature Sources