Metalloproteinases and Wound Healing
- PMID: 25945285
- PMCID: PMC4397992
- DOI: 10.1089/wound.2014.0581
Metalloproteinases and Wound Healing
Abstract
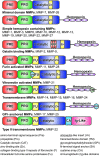
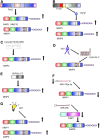
Significance: Matrix metalloproteinases (MMPs) are present in both acute and chronic wounds. They play a pivotal role, with their inhibitors, in regulating extracellular matrix degradation and deposition that is essential for wound reepithelialization. The excess protease activity can lead to a chronic nonhealing wound. The timed expression and activation of MMPs in response to wounding are vital for successful wound healing. MMPs are grouped into eight families and display extensive homology within these families. This homology leads in part to the initial failure of MMP inhibitors in clinical trials and the development of alternative methods for modulating the MMP activity. MMP-knockout mouse models display altered wound healing responses, but these are often subtle phenotypic changes indicating the overlapping MMP substrate specificity and inter-MMP compensation. Recent Advances: Recent research has identified several new MMP modulators, including photodynamic therapy, protease-absorbing dressing, microRNA regulation, signaling molecules, and peptides. Critical Issues: Wound healing requires the controlled activity of MMPs at all stages of the wound healing process. The loss of MMP regulation is a characteristic of chronic wounds and contributes to the failure to heal. Future Directions: Further research into how MMPs are regulated should allow the development of novel treatments for wound healing.
Figures


References
-
- Zhang X, Nothnick WB. The role and regulation of the uterine matrix metalloproteinase system in menstruating and non-menstruating species. Front Biosci 2005;10:353–366 - PubMed
-
- Steffensen B, Hakkinen L, Larjava H. Proteolytic events of wound-healing—coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med 2001;12:373–398 - PubMed
-
- Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol 2007;211:19–26 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

