Laminins: Roles and Utility in Wound Repair
- PMID: 25945287
- PMCID: PMC4397997
- DOI: 10.1089/wound.2014.0533
Laminins: Roles and Utility in Wound Repair
Abstract
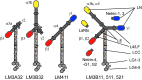
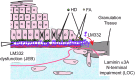
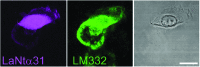
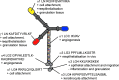
Significance: Laminins are complex extracellular macromolecules that are major players in the control of a variety of core cell processes, including regulating rates of cell proliferation, differentiation, adhesion, and migration. Laminins, and related extracellular matrix components, have essential roles in tissue homeostasis; however, during wound healing, the same proteins are critical players in re-epithelialization and angiogenesis. Understanding how these proteins influence cell behavior in these different conditions holds great potential in identifying new strategies to enhance normal wound closure or to treat chronic/nonhealing wounds. Recent Advances: Laminin-derived bioactive peptides and, more recently, laminin-peptide conjugated scaffolds, have been designed to improve tissue regeneration after injuries. These peptides have been shown to be effective in decreasing inflammation and granulation tissue, and in promoting re-epithelialization, angiogenesis, and cell migration. Critical Issues: Although there is now a wealth of knowledge concerning laminin form and function, there are still areas of some controversy. These include the relative contribution of two laminin-based adhesive devices (focal contacts and hemidesmosomes) to the re-epithelialization process, the impact and implications of laminin proteolytic processing, and the importance of laminin polymer formation on cell behavior. In addition, the roles in wound healing of the laminin-related proteins, netrins, and LaNts are still to be fully defined. Future Directions: The future of laminin-based therapeutics potentially lies in the bioengineering of specific substrates to support laminin deposition for ex vivo expansion of autologous cells for graft formation and transplantation. Significant recent advances suggest that this goal is within sight.
Figures


References
-
- Kivirikko S, McGrath JA, Pulkkinen L, Uitto J, Christiano AM. Mutational hotspots in the LAMB3 gene in the lethal (Herlitz) type of junctional epidermolysis bullosa. Hum Mol Genet 1996;5:231–237 - PubMed
-
- Muhle C, Jiang QJ, Charlesworth A, Bruckner-Tuderman L, Meneguzzi G, Schneider H. Novel and recurrent mutations in the laminin-5 genes causing lethal junctional epidermolysis bullosa: molecular basis and clinical course of Herlitz disease. Hum Genet 2005;116:33–42 - PubMed
-
- Pulkkinen L, Christiano AM, Airenne T, Haakana H, Tryggvason K, Uitto J. Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet 1994;6:293–297 - PubMed
-
- Ko MS, Marinkovich MP. Role of dermal-epidermal basement membrane zone in skin, cancer, and developmental disorders. Dermatol Clin 2010;28:1–16 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

