Shaped singular spectrum analysis for quantifying gene expression, with application to the early Drosophila embryo
- PMID: 25945341
- PMCID: PMC4402483
- DOI: 10.1155/2015/689745
Shaped singular spectrum analysis for quantifying gene expression, with application to the early Drosophila embryo
Abstract
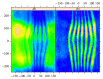
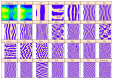
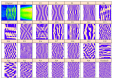
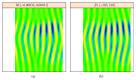
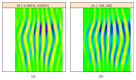
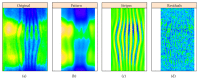
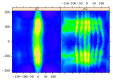
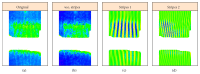
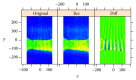
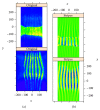
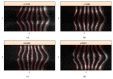
In recent years, with the development of automated microscopy technologies, the volume and complexity of image data on gene expression have increased tremendously. The only way to analyze quantitatively and comprehensively such biological data is by developing and applying new sophisticated mathematical approaches. Here, we present extensions of 2D singular spectrum analysis (2D-SSA) for application to 2D and 3D datasets of embryo images. These extensions, circular and shaped 2D-SSA, are applied to gene expression in the nuclear layer just under the surface of the Drosophila (fruit fly) embryo. We consider the commonly used cylindrical projection of the ellipsoidal Drosophila embryo. We demonstrate how circular and shaped versions of 2D-SSA help to decompose expression data into identifiable components (such as trend and noise), as well as separating signals from different genes. Detection and improvement of under- and overcorrection in multichannel imaging is addressed, as well as the extraction and analysis of 3D features in 3D gene expression patterns.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

