IgM exacerbates glomerular disease progression in complement-induced glomerulopathy
- PMID: 25945405
- PMCID: PMC4556608
- DOI: 10.1038/ki.2015.120
IgM exacerbates glomerular disease progression in complement-induced glomerulopathy
Abstract
Although glomerular immunoglobulin M (IgM) deposition occurs in a variety of glomerular diseases, the mechanism of deposition and its clinical significance remain controversial. Some have theorized IgM becomes passively trapped in areas of glomerulosclerosis. However, recent studies found that IgM specifically binds damaged glomeruli. Therefore, we tested whether natural IgM binds to neo-epitopes exposed after insults to the glomerulus and exacerbates disease in mice deficient in the complement regulatory protein factor H; a model of non-sclerotic and nonimmune-complex glomerular disease. Immunofluorescence microscopy demonstrated mesangial and capillary loop deposition of IgM, whereas ultrastructural analysis found IgM deposition on endothelial cells and subendothelial areas. Factor H-deficient mice lacking B cells were protected from renal damage, as evidenced by milder histologic lesions on light and electron microscopy. IgM, but not IgG, from wild-type mice bound to cultured murine mesangial cells. Furthermore, injection of purified IgM into mice lacking B cells bound within the glomeruli and induced proteinuria. A monoclonal natural IgM-recognizing phospholipids also bound to glomeruli in vivo and induced albuminuria. Thus, our results indicate specific IgM antibodies bind to glomerular epitopes and that IgM contributes to the progression of glomerular damage in this mouse model of non-sclerotic glomerular disease.
Figures
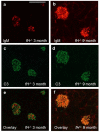
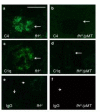
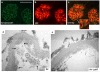
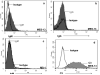

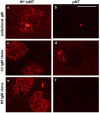

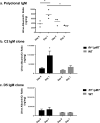
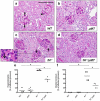

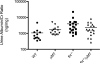
Comment in
-
IgM in the kidney: a multiple personality disorder.Kidney Int. 2015 Sep;88(3):439-41. doi: 10.1038/ki.2015.153. Kidney Int. 2015. PMID: 26323070 Free PMC article.
References
-
- Habib R, Girardin E, Gagnadoux MF, et al. Immunopathological findings in idiopathic nephrosis: clinical significance of glomerular “immune deposits”. Pediatr Nephrol. 1988;2:402–408. - PubMed
-
- Gephardt GN, Tubbs RR, Popowniak KL, et al. Focal and segmental glomerulosclerosis. Immunohistologic study of 20 renal biopsy specimens. Arch Pathol Lab Med. 1986;110:902–905. - PubMed
-
- Mujais SK, Emmanouel DS, Kasinath BS, et al. Marked proteinuria in hypertensive nephrosclerosis. American journal of nephrology. 1985;5:190–195. - PubMed
-
- Ainsworth SK, Hirsch HZ, Brackett NC, Jr., et al. Diabetic glomerulonephropathy: histopathologic, immunofluorescent, and ultrastructural studies of 16 cases. Human pathology. 1982;13:470–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

