Regulating the stability and localization of CDK inhibitor p27(Kip1) via CSN6-COP1 axis
- PMID: 25945542
- PMCID: PMC4613181
- DOI: 10.1080/15384101.2015.1046655
Regulating the stability and localization of CDK inhibitor p27(Kip1) via CSN6-COP1 axis
Abstract
The COP9 signalosome subunit 6 (CSN6), which is involved in ubiquitin-mediated protein degradation, is overexpressed in many types of cancer. CSN6 is critical in causing p53 degradation and malignancy, but its target in cell cycle progression is not fully characterized. Constitutive photomorphogenic 1 (COP1) is an E3 ubiquitin ligase associating with COP9 signalosome to regulate important target proteins for cell growth. p27 is a critical G1 CDK inhibitor involved in cell cycle regulation, but its upstream regulators are not fully characterized. Here, we show that the CSN6-COP1 link is regulating p27(Kip1) stability, and that COP1 is a negative regulator of p27(Kip1). Ectopic expression of CSN6 can decrease the expression of p27(Kip1), while CSN6 knockdown leads to p27(Kip1) stabilization. Mechanistic studies show that CSN6 interacts with p27(Kip1) and facilitates ubiquitin-mediated degradation of p27(Kip1). CSN6-mediated p27 degradation depends on the nuclear export of p27(Kip1), which is regulated through COP1 nuclear exporting signal. COP1 overexpression leads to the cytoplasmic distribution of p27, thereby accelerating p27 degradation. Importantly, the negative impact of COP1 on p27 stability contributes to elevating expression of genes that are suppressed through p27 mediation. Kaplan-Meier analysis of tumor samples demonstrates that high COP1 expression was associated with poor overall survival. These data suggest that tumors with CSN6/COP1 deregulation may have growth advantage by regulating p27 degradation and subsequent impact on p27 targeted genes.
Keywords: COP1; COP9 signalosome; CSN6; p27; ubiquitination.
Figures



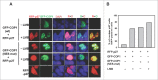


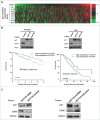
Similar articles
-
CSN6 deregulation impairs genome integrity in a COP1-dependent pathway.Oncotarget. 2015 May 20;6(14):11779-93. doi: 10.18632/oncotarget.3151. Oncotarget. 2015. PMID: 25957415 Free PMC article.
-
COP9 signalosome subunit 6 stabilizes COP1, which functions as an E3 ubiquitin ligase for 14-3-3σ.Oncogene. 2011 Dec 1;30(48):4791-801. doi: 10.1038/onc.2011.192. Epub 2011 May 30. Oncogene. 2011. PMID: 21625211 Free PMC article.
-
CSN6 controls the proliferation and metastasis of glioblastoma by CHIP-mediated degradation of EGFR.Oncogene. 2017 Feb 23;36(8):1134-1144. doi: 10.1038/onc.2016.280. Epub 2016 Aug 22. Oncogene. 2017. PMID: 27546621
-
Constitutive photomorphogenesis protein 1 (COP1) and COP9 signalosome, evolutionarily conserved photomorphogenic proteins as possible targets of melatonin.J Pineal Res. 2016 Aug;61(1):41-51. doi: 10.1111/jpi.12340. Epub 2016 Jun 13. J Pineal Res. 2016. PMID: 27121162 Review.
-
Roles of COP9 signalosome in cancer.Cell Cycle. 2011 Sep 15;10(18):3057-66. doi: 10.4161/cc.10.18.17320. Epub 2011 Sep 15. Cell Cycle. 2011. PMID: 21876386 Free PMC article. Review.
Cited by
-
Targeting RFWD2 as an Effective Strategy to Inhibit Cellular Proliferation and Overcome Drug Resistance to Proteasome Inhibitor in Multiple Myeloma.Front Cell Dev Biol. 2021 Apr 21;9:675939. doi: 10.3389/fcell.2021.675939. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33968945 Free PMC article.
-
EGF Relays Signals to COP1 and Facilitates FOXO4 Degradation to Promote Tumorigenesis.Adv Sci (Weinh). 2020 Sep 23;7(20):2000681. doi: 10.1002/advs.202000681. eCollection 2020 Oct. Adv Sci (Weinh). 2020. PMID: 33101846 Free PMC article.
-
Correlation of constitutive photomorphogenic 1 (COP1) and p27 tumor suppressor protein expression in ovarian cancer.Genes Genomics. 2019 Aug;41(8):879-884. doi: 10.1007/s13258-019-00818-6. Epub 2019 Apr 26. Genes Genomics. 2019. PMID: 31028655
-
The DenA/DEN1 Interacting Phosphatase DipA Controls Septa Positioning and Phosphorylation-Dependent Stability of Cytoplasmatic DenA/DEN1 during Fungal Development.PLoS Genet. 2016 Mar 24;12(3):e1005949. doi: 10.1371/journal.pgen.1005949. eCollection 2016 Mar. PLoS Genet. 2016. PMID: 27010942 Free PMC article.
-
CSN6 Promotes the Migration and Invasion of Cervical Cancer Cells by Inhibiting Autophagic Degradation of Cathepsin L.Int J Biol Sci. 2019 May 12;15(6):1310-1324. doi: 10.7150/ijbs.32987. eCollection 2019. Int J Biol Sci. 2019. PMID: 31223289 Free PMC article.
References
-
- Wei N, Deng XW. Making sense of the COP9 signalosome. A regulatory protein complex conserved from Arabidopsis to human. Trends Genet 1999; 15:98-103; PMID:10203806; http://dx.doi.org/10.1016/S0168-9525(98)01670-9 - DOI - PubMed
-
- Seeger M, Kraft R, Ferrell K, Bech-Otschir D, Dumdey R, Schade R, Gordon C, Naumann M, Dubiel W. A novel protein complex involved in signal transduction possessing similarities to 26S proteasome subunits. FASEB J 1998; 12:469-78; PMID:9535219 - PubMed
-
- Zhang XC, Chen J, Su CH, Yang HY, Lee MH. Roles for CSN5 in control of p53/MDM2 activities. J Cell Biochem 2008; 103:1219-30; PMID:17879958; http://dx.doi.org/10.1002/jcb.21504 - DOI - PubMed
-
- Chen B, Zhao R, Su CH, Linan M, Tseng C, Phan L, Fang L, Yang HY, Yang H, Wang W, et al.. CDK inhibitor p57 (Kip2) is negatively regulated by COP9 signalosome subunit 6. Cell Cycle 2012; 11:4633-41; PMID:23187808; http://dx.doi.org/10.4161/cc.22887 - DOI - PMC - PubMed
-
- Xue Y, Chen J, Choi HH, Phan L, Chou PC, Zhao R, Yang H, Santiago J, Liu M, Yeung GE, et al.. HER2-Akt signaling in regulating COP9 signalsome subunit 6 and p53. Cell Cycle 2012; 11:4181-90; PMID:23095642; http://dx.doi.org/10.4161/cc.22413 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
