Structural Basis Underlying the Binding Preference of Human Galectins-1, -3 and -7 for Galβ1-3/4GlcNAc
- PMID: 25945972
- PMCID: PMC4422656
- DOI: 10.1371/journal.pone.0125946
Structural Basis Underlying the Binding Preference of Human Galectins-1, -3 and -7 for Galβ1-3/4GlcNAc
Abstract
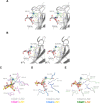
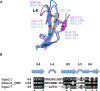
Galectins represent β-galactoside-binding proteins and are known to bind Galβ1-3/4GlcNAc disaccharides (abbreviated as LN1 and LN2, respectively). Despite high sequence and structural homology shared by the carbohydrate recognition domain (CRD) of all galectin members, how each galectin displays different sugar-binding specificity still remains ambiguous. Herein we provided the first structural evidence of human galectins-1, 3-CRD and 7 in complex with LN1. Galectins-1 and 3 were shown to have higher affinity for LN2 than for LN1, while galectin-7 displayed the reversed specificity. In comparison with the previous LN2-complexed structures, the results indicated that the average glycosidic torsion angle of galectin-bound LN1 (ψ(LN1) ≈ 135°) was significantly differed from that of galectin-bound LN2 (ψ(LN2 )≈ -108°), i.e. the GlcNAc moiety adopted a different orientation to maintain essential interactions. Furthermore, we also identified an Arg-Asp/Glu-Glu-Arg salt-bridge network and the corresponding loop (to position the second Asp/Glu residue) critical for the LN1/2-binding preference.
Conflict of interest statement
Figures



References
-
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994. February 25;76(4):597–8. - PubMed
-
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconjugate journal. 2004;19(7–9):433–40. - PubMed
-
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005. January;5(1):29–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

