The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans
- PMID: 25946076
- PMCID: PMC4496692
- DOI: 10.3390/biom5020702
The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans
Abstract
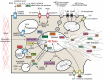
Extracellular traps (ETs) are reticulate structures of extracellular DNA associated with antimicrobial molecules. Their formation by phagocytes (mainly by neutrophils: NETs) has been identified as an essential element of vertebrate innate immune defense. However, as ETs are also toxic to host cells and potent triggers of autoimmunity, their role between pathogen defense and human pathogenesis is ambiguous, and they contribute to a variety of acute and chronic inflammatory diseases. Since the discovery of ET formation (ETosis) a decade ago, evidence has accumulated that most reaction cascades leading to ET release involve ROS. An important new facet was added when it became apparent that ETosis might be directly linked to, or be a variant of, the autophagy cell death pathway. The present review analyzes the evidence to date on the interplay between ROS, autophagy and ETosis, and highlights and discusses several further aspects of the ROS-ET relationship that are incompletely understood. These aspects include the role of NADPH oxidase-derived ROS, the molecular requirements of NADPH oxidase-dependent ETosis, the roles of NADPH oxidase subtypes, extracellular ROS and of ROS from sources other than NADPH oxidase, and the present evidence for ROS-independent ETosis. We conclude that ROS interact with ETosis in a multidimensional manner, with influence on whether ETosis shows beneficial or detrimental effects.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

