Identification of CTLA2A, DEFB29, WFDC15B, SERPINA1F and MUP19 as Novel Tissue-Specific Secretory Factors in Mouse
- PMID: 25946105
- PMCID: PMC4422522
- DOI: 10.1371/journal.pone.0124962
Identification of CTLA2A, DEFB29, WFDC15B, SERPINA1F and MUP19 as Novel Tissue-Specific Secretory Factors in Mouse
Abstract
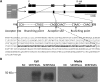
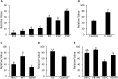
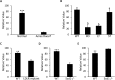
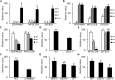
Secretory factors in animals play an important role in communication between different cells, tissues and organs. Especially, the secretory factors with specific expression in one tissue may reflect important functions and unique status of that tissue in an organism. In this study, we identified potential tissue-specific secretory factors in the fat, muscle, heart, lung, kidney and liver in the mouse by analyzing microarray data from NCBI's Gene Expression Omnibus (GEO) public repository and searching and predicting their subcellular location in GeneCards and WoLF PSORT, and then confirmed tissue-specific expression of the genes using semi-quantitative PCR reactions. With this approach, we confirmed 11 lung, 7 liver, 2 heart, 1 heart and muscle, 7 kidney and 2 adipose and liver-specific secretory factors. Among these genes, 1 lung-specific gene--CTLA2A (cytotoxic T lymphocyte-associated protein 2 alpha), 3 kidney-specific genes--SERPINA1F (serpin peptidase inhibitor, Clade A, member 1F), WFDC15B (WAP four-disulfide core domain 15B) and DEFB29 (defensin beta 29) and 1 liver-specific gene--MUP19 (major urinary protein 19) have not been reported as secretory factors. These genes were tagged with hemagglutinin at the 3'end and then transiently transfected to HEK293 cells. Through protein detection in cell lysate and media using Western blotting, we verified secretion of the 5 genes and predicted the potential pathways in which they may participate in the specific tissue through data analysis of GEO profiles. In addition, alternative splicing was detected in transcripts of CTLA2A and SERPINA1F and the corresponding proteins were found not to be secreted in cell culture media. Identification of novel secretory factors through the current study provides a new platform to explore novel secretory factors and a general direction for further study of these genes in the future.
Conflict of interest statement
Figures



Similar articles
-
Identification of novel tissue-specific genes by analysis of microarray databases: a human and mouse model.PLoS One. 2013 May 31;8(5):e64483. doi: 10.1371/journal.pone.0064483. Print 2013. PLoS One. 2013. PMID: 23741331 Free PMC article.
-
Identification and characterization of novel and unknown mouse epididymis-specific genes by complementary DNA microarray technology.Biol Reprod. 2006 Sep;75(3):462-8. doi: 10.1095/biolreprod.105.048058. Epub 2006 May 17. Biol Reprod. 2006. PMID: 16707773
-
MGFM: a novel tool for detection of tissue and cell specific marker genes from microarray gene expression data.BMC Genomics. 2015 Aug 28;16(1):645. doi: 10.1186/s12864-015-1785-9. BMC Genomics. 2015. PMID: 26314578 Free PMC article.
-
Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase.Reprod Biol Endocrinol. 2004 Jul 7;2:54. doi: 10.1186/1477-7827-2-54. Reprod Biol Endocrinol. 2004. PMID: 15239838 Free PMC article.
-
Role of skeletal muscle in mandible development.Histol Histopathol. 2014 Nov;29(11):1377-94. doi: 10.14670/HH-29.1377. Epub 2014 May 27. Histol Histopathol. 2014. PMID: 24867377 Review.
Cited by
-
Single-cell RNA sequencing reveals placental response under environmental stress.Nat Commun. 2024 Aug 2;15(1):6549. doi: 10.1038/s41467-024-50914-9. Nat Commun. 2024. PMID: 39095385 Free PMC article.
-
Identification of Smad3-related transcriptomes in type-2 diabetic nephropathy by whole transcriptome RNA sequencing.J Cell Mol Med. 2021 Feb;25(4):2052-2068. doi: 10.1111/jcmm.16133. Epub 2020 Dec 25. J Cell Mol Med. 2021. PMID: 33369170 Free PMC article.
-
LRG1: an emerging player in disease pathogenesis.J Biomed Sci. 2022 Jan 21;29(1):6. doi: 10.1186/s12929-022-00790-6. J Biomed Sci. 2022. PMID: 35062948 Free PMC article. Review.
-
Interaction of the Mineralocorticoid Receptor With RACK1 and Its Role in Aldosterone Signaling.Endocrinology. 2017 Jul 1;158(7):2367-2375. doi: 10.1210/en.2017-00095. Endocrinology. 2017. PMID: 28472300 Free PMC article.
-
Identification of the MUC2 Promoter as a Strong Promoter for Intestinal Gene Expression through Generation of Transgenic Quail Expressing GFP in Gut Epithelial Cells.Int J Mol Sci. 2017 Jan 19;18(1):196. doi: 10.3390/ijms18010196. Int J Mol Sci. 2017. PMID: 28106824 Free PMC article.
References
-
- Corsi AK, Schekman R. Mechanism of polypeptide translocation into the endoplasmic reticulum. J Biol Chem. 1996; 271: 30299–30302. - PubMed
-
- Glick B, Malhotra V. The curious status of the Golgi apparatus. Cell. 1988; 95:883–889. - PubMed
-
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Section 17.3, Overview of the Secretory Pathway In: Molecular Cell Biology. 4th ed. New York: W. H. Freeman; 2000. Available: http://www.ncbi.nlm.nih.gov/books/NBK21471/.
-
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372: 425–432. - PubMed
-
- Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005; 64: 163–169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

