The efficacy of 2-nitrovinylfuran derivatives against Leishmania in vitro and in vivo
- PMID: 25946239
- PMCID: PMC4489446
- DOI: 10.1590/0074-02760140324
The efficacy of 2-nitrovinylfuran derivatives against Leishmania in vitro and in vivo
Abstract
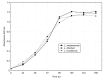
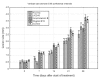
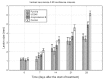
Despite recent advances in the treatment of some forms of leishmaniasis, the available drugs are still far from ideal due to inefficacy, parasite resistance, toxicity and cost. The wide-spectrum antimicrobial activity of 2-nitrovinylfuran compounds has been described, as has their activity against Trichomonas vaginalis and other protozoa. Thus, the aim of this study was to test the antileishmanial activities of six 2-nitrovinylfurans in vitro and in a murine model of leishmaniasis. Minimum parasiticide concentration (MPC) and 50% inhibitory concentration (IC50) values for these compounds against the promastigotes of Leishmania amazonensis, Leishmania infantum and Leishmania braziliensis were determined, as were the efficacies of two selected compounds in an experimental model of cutaneous leishmaniasis (CL) caused by L. amazonensis in BALB/c mice. All of the compounds were active against the promastigotes of the three Leishmania species tested. IC50 and MPC values were in the ranges of 0.8-4.7 µM and 1.7-32 µM, respectively. The compounds 2-bromo-5-(2-bromo-2-nitrovinyl)-furan (furvina) and 2-bromo-5-(2-methyl-2-nitrovinyl)-furan (UC245) also reduced lesion growth in vivo at a magnitude comparable to or higher than that achieved by amphotericin B treatment. The results demonstrate the potential of this class of compounds as antileishmanial agents and support the clinical testing of Dermofural(r) (a furvina-containing antifungal ointment) for the treatment of CL.
Figures

References
-
- Alabi KA, Owolabi BJ. Synthesis and antimicrobial property of 2-(2-nitrovinyl)furan. J Pure Appl Microbiol. 2012;6:131–134.
-
- AlSamarai AM, AlObaidi HS. Cutaneous leishmaniasis in Iraq. J Infect Dev Ctries. 2009;3:123–129. - PubMed
-
- Bellenghi M, Wittgens A. The antimycotic and antibacterial effectiveness of acryl and nitrovinyl derivatives of benzene, thiophene and furan series. Arzneimittelforschung. 1958;8:263–268. - PubMed
-
- Blondeau JM, Castañedo N, González O, Mendina R, Silveira E. In vitro evaluation of G1: a novel antimicrobial compound. Int J Antimicrob Agents. 1999;11:163–166. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

