SYK regulates macrophage MHC-II expression via activation of autophagy in response to oxidized LDL
- PMID: 25946330
- PMCID: PMC4509444
- DOI: 10.1080/15548627.2015.1037061
SYK regulates macrophage MHC-II expression via activation of autophagy in response to oxidized LDL
Abstract
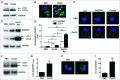
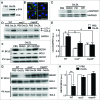
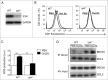
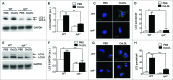
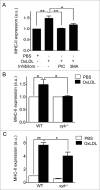
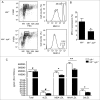
Adaptive immunity, which plays an important role in the development of atherosclerosis, is mediated by major histocompatibility complex (MHC)-dependent antigen presentation. In atherosclerotic lesions, macrophages constitute an important class of antigen-presenting cells that activate adaptive immune responses to oxidized low-density lipoprotein (OxLDL). It has been reported that autophagy regulates adaptive immune responses by enhancing antigen presentation to MHC class II (MHC-II). In a previous study, we have demonstrated that SYK (spleen tyrosine kinase) regulates generation of reactive oxygen species (ROS) and activation of MAPK8/JNK1 in macrophages. Because ROS and MAPK8 are known to regulate autophagy, in this study we investigated the role of SYK in autophagy, MHC-II expression and adaptive immune response to OxLDL. We demonstrate that OxLDL induces autophagosome formation, MHC-II expression, and phosphorylation of SYK in macrophages. Gene knockout and pharmacological inhibitors of NOX2 and MAPK8 reduced OxLDL-induced autophagy. Using bone marrow-derived macrophages isolated from wild-type and myeloid-specific SYK knockout mice, we demonstrate that SYK regulates OxLDL-induced ROS generation, MAPK8 activation, BECN1-BCL2 dissociation, autophagosome formation and presentation of OxLDL-derived antigens to CD4(+) T cells. ldlr(-/-) syk(-/-) mice fed a high-fat diet produced lower levels of IgG to malondialdehyde (MDA)-LDL, malondialdehyde-acetaldehyde (MAA)-LDL, and OxLDL compared to ldlr(-/-) mice. These results provide new insights into the mechanisms by which SYK regulates MHC-II expression via autophagy in macrophages and may contribute to regulation of adaptive immune responses in atherosclerosis.
Keywords: 3MA, 3-methyladenine; APCs, antigen-presenting cells; BCR, B cell receptor; BMDM, bone marrow-derived macrophage; Baf, bafilomycin A1; DPI, diphenyleneiodonium; FCGR, Fc fragment of IgG; GFP, green fluorescent protein; HFD, high-fat diet; IL2, interleukin 2; ITAM, immunoreceptor tyrosine-based activation motif; IgG, immunoglobulin G; IgM, immunoglobulin M; LPS, lipopolysaccharide; MAA-LDL, malondialdehyde-acetaldehyde modified low density lipoprotein; MAP1LC3/LC3, microtubule-associated protein 1 light chain 3; MAPK, mitogen-activated protein kinase; MDA-LDL, malondialdehyde modified low density lipoprotein; MHC-II; MHC-II, major histocompatibility complex class II; NOX, NAPDH oxidase; OSE, oxidation specific epitopes; OxLDL; OxLDL, oxidized low density lipoprotein; PBS, phosphate-buffered saline; PIC, piceatannol; ROS; ROS, reactive oxygen species; SYK; SYK, spleen tyrosine kinase; TCR, T cell receptor; TLR4, toll-like receptor 4; TNF, tumor necrosis factor; autophagy; low affinity, receptor; mmLDL, minimally modified low density lipoprotein; oxidation-specific antibodies.
Figures


References
-
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006; 6:508-19; PMID:16778830; http://dx.doi.org/10.1038/nri1882 - DOI - PubMed
-
- Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest 2013; 123:27-36; PMID:23281407; http://dx.doi.org/10.1172/JCI63108 - DOI - PMC - PubMed
-
- Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98:800-14; PMID:8698873; http://dx.doi.org/10.1172/JCI118853 - DOI - PMC - PubMed
-
- Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 2004; 114:427-37; PMID:15286809; http://dx.doi.org/10.1172/JCI200420479 - DOI - PMC - PubMed
-
- Gonen A, Hansen LF, Turner WW, Montano EN, Que X, Rafia A, Chou MY, Wiesner P, Tsiantoulas D, Corr M, et al.. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. J Lipid Res 2014; 55:2137-55; PMID:25143462; http://dx.doi.org/10.1194/jlr.M053256 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
