Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer
- PMID: 25947066
- PMCID: PMC4504229
- DOI: 10.1186/s12967-015-0511-3
Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer
Abstract
Background: Fatty acid synthase (FASN) is crucial to de novo long-chain fatty acid synthesis, needed to meet cancer cells' increased demands for membrane, energy, and protein production.
Methods: We investigated FASN overexpression as a therapeutic and chemosensitization target in ovarian cancer tissue, cell lines, and primary cell cultures. FASN expression at mRNA and protein levels was determined by quantitative real-time polymerase chain reaction and immunoblotting and immunohistochemistry, respectively. FASN inhibition's impact on cell viability, apoptosis, and fatty acid metabolism was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide assay, cell death detection enzyme-linked immunosorbent assay, immunoblotting, and (18) F-fluoromethylcholine uptake measurement, respectively.
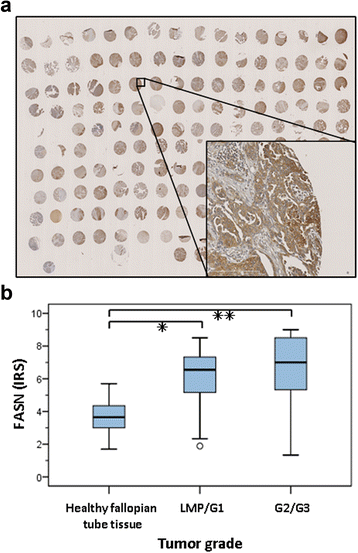
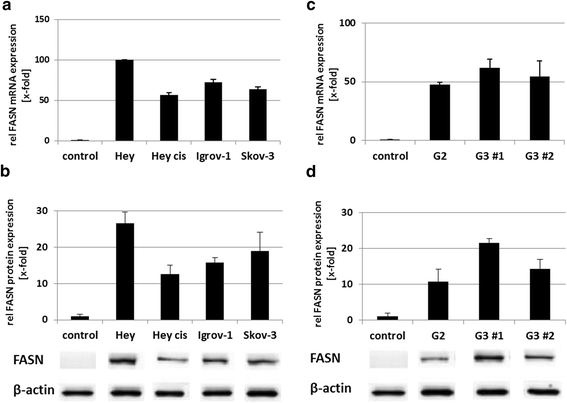
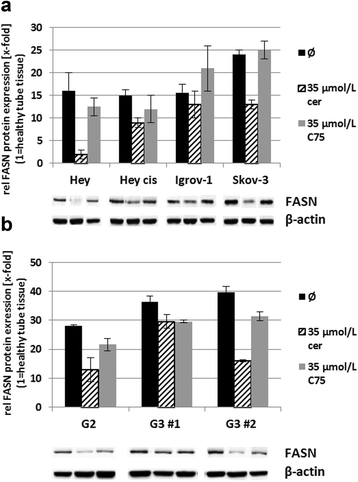
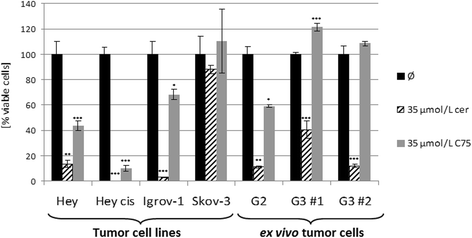
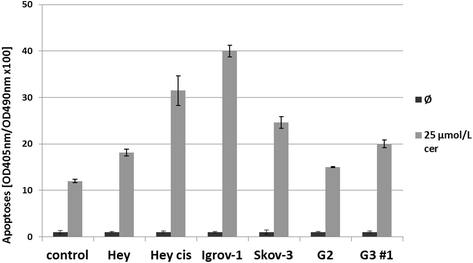
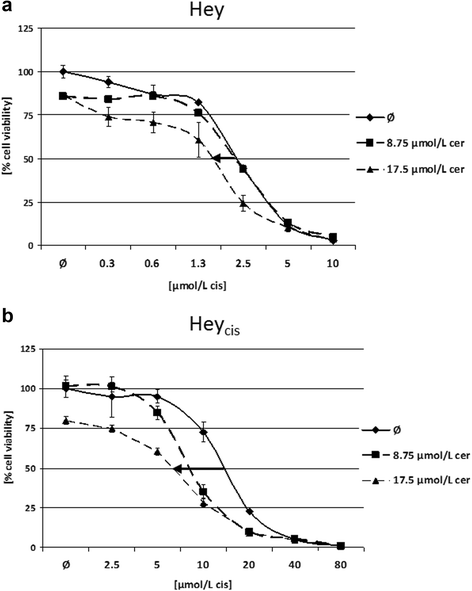
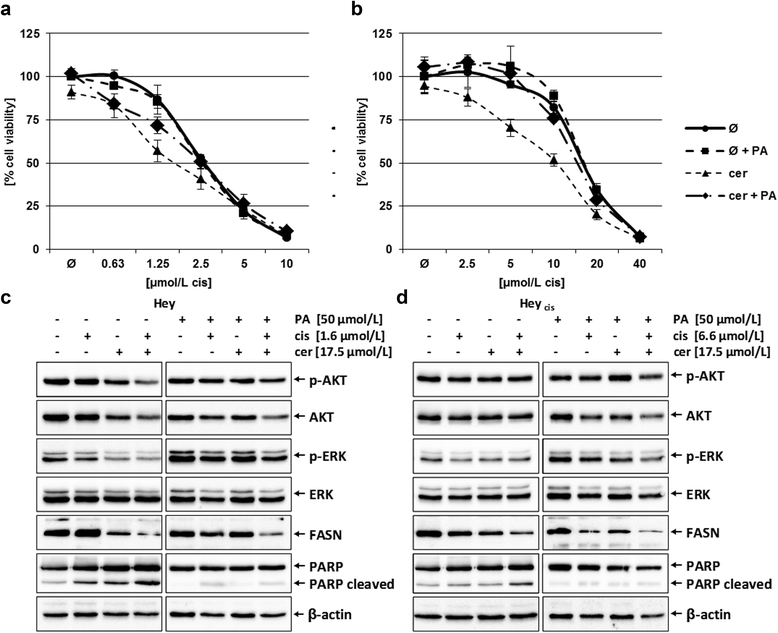
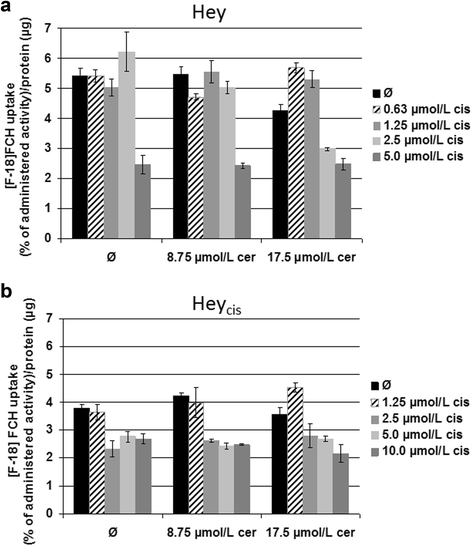
Results: Relative to that in healthy fallopian tube tissue, tumor tissues had 1.8-fold average FASN protein overexpression; cell lines and primary cultures had 11-fold-100-fold mRNA and protein overexpression. In most samples, the FASN inhibitor cerulenin markedly decreased FASN expression and cell viability and induced apoptosis. Unlike concomitant administration, sequential cerulenin/cisplatin treatment reduced cisplatin's half maximal inhibitory concentration profoundly (up to 54%) in a cisplatin-resistant cell line, suggesting platinum (re)sensitization. Cisplatin-resistant cells displayed lower (18) F-fluoro-methylcholine uptake than did cisplatin-sensitive cells, suggesting that metabolic imaging might help guide therapy.
Conclusions: FASN inhibition induced apoptosis in chemosensitive and platinum-resistant ovarian cancer cells and may reverse cisplatin resistance.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

