Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc
- PMID: 25947151
- PMCID: PMC4443361
- DOI: 10.1073/pnas.1503348112
Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc
Abstract
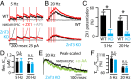
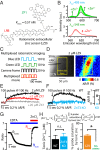
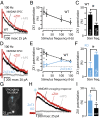
Many excitatory synapses contain high levels of mobile zinc within glutamatergic vesicles. Although synaptic zinc and glutamate are coreleased, it is controversial whether zinc diffuses away from the release site or whether it remains bound to presynaptic membranes or proteins after its release. To study zinc transmission and quantify zinc levels, we required a high-affinity rapid zinc chelator as well as an extracellular ratiometric fluorescent zinc sensor. We demonstrate that tricine, considered a preferred chelator for studying the role of synaptic zinc, is unable to efficiently prevent zinc from binding low-nanomolar zinc-binding sites, such as the high-affinity zinc-binding site found in NMDA receptors (NMDARs). Here, we used ZX1, which has a 1 nM zinc dissociation constant and second-order rate constant for binding zinc that is 200-fold higher than those for tricine and CaEDTA. We find that synaptic zinc is phasically released during action potentials. In response to short trains of presynaptic stimulation, synaptic zinc diffuses beyond the synaptic cleft where it inhibits extrasynaptic NMDARs. During higher rates of presynaptic stimulation, released glutamate activates additional extrasynaptic NMDARs that are not reached by synaptically released zinc, but which are inhibited by ambient, tonic levels of nonsynaptic zinc. By performing a ratiometric evaluation of extracellular zinc levels in the dorsal cochlear nucleus, we determined the tonic zinc levels to be low nanomolar. These results demonstrate a physiological role for endogenous synaptic as well as tonic zinc in inhibiting extrasynaptic NMDARs and thereby fine tuning neuronal excitability and signaling.
Keywords: NMDA receptors; glutamate spillover; ratiometric zinc sensors; zinc; zinc chelators; zinc dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

