DCC functions as an accelerator of thalamocortical axonal growth downstream of spontaneous thalamic activity
- PMID: 25947198
- PMCID: PMC4515124
- DOI: 10.15252/embr.201439882
DCC functions as an accelerator of thalamocortical axonal growth downstream of spontaneous thalamic activity
Abstract
Controlling the axon growth rate is fundamental when establishing brain connections. Using the thalamocortical system as a model, we previously showed that spontaneous calcium activity influences the growth rate of thalamocortical axons by regulating the transcription of Robo1 through an NF-κB-binding site in its promoter. Robo1 acts as a brake on the growth of thalamocortical axons in vivo. Here, we have identified the Netrin-1 receptor DCC as an accelerator for thalamic axon growth. Dcc transcription is regulated by spontaneous calcium activity in thalamocortical neurons and activating DCC signaling restores normal axon growth in electrically silenced neurons. Moreover, we identified an AP-1-binding site in the Dcc promoter that is crucial for the activity-dependent regulation of this gene. In summary, we have identified the Dcc gene as a novel downstream target of spontaneous calcium activity involved in axon growth. Together with our previous data, we demonstrate a mechanism to control axon growth that relies on the activity-dependent regulation of two functionally opposed receptors, Robo1 and DCC. These two proteins establish a tight and efficient means to regulate activity-guided axon growth in order to correctly establish neuronal connections during development.
Keywords: Netrin‐1/Dcc signalling; axon growth; development; spontaneous activity; thalamus.
© 2015 The Authors.
Figures
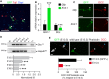
Embryonic dissociated thalamic neurons transfected with Gfp (green) and immunostained for Tuj1 (red). Nuclear counterstaining with DAPI is shown in blue. Scale bar, 100 μm. Arrowheads indicate examples of Gfp-transfected cells labeled by Tuj1.
Real-time PCR quantification of the transcripts for Kir2.1 and Dcc in Gfp- and Kir2.1-transfected cells: ***P < 0.001, Mann–Whitney U-test. The data are presented as the means ± s.e.m.
Immunocytochemistry for DCC in control and Kir2.1-expressing axons. Less DCC expression was found in growth cones transfected with Kir2.1 compared to controls (arrowheads). Scale bar, 10 μm.
Semi-quantitative PCR for Dcc and gapdh, normalizing Dcc expression to that of gapdh: ***P < 0.001, one-way ANOVA with Tukey's post hoc analysis. ID, integrated density. The data are presented as the means ± s.e.m.
Immunocytochemistry for DCC in E13.5 (GFP-positive; white arrowhead) and E15.5 (GFP-negative; blue arrowhead) isolated thalamic growth cones. Thalamic explants from E13.5 GFP mouse were cocultured together with explants from wild-type E15.5 embryos on polylysine/laminin coverslips. Scale bar, 10 μm. Quantification of the data shown: ***P < 0.001, two-tailed Student's t-test. ID, integrated density. The data are presented as the means ± s.e.m.
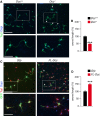
Tuj1 immunostaining revealed that thalamic axon growth decreased in the absence of DCC (arrowheads). Scale bars: 100 μm lower power panels; and 50 μm, higher power panels.
Quantification of the data shown in A. ***P < 0.001, two-tailed Student's t-test. The data are presented as the means ± s.e.m.
Tuj1 staining revealed an increase in thalamic axon growth following Dcc overexpression with a full-length Dcc (FL-Dcc) construct (arrowheads). Scale bars: 100 μm, lower power panels; and 50 μm, higher power panels.
Quantification of the data in C. When DCC levels were elevated, axon growth increased significantly when compared to control conditions. ***P < 0.001, two-tailed Student's t-test. The data are presented as the means ± s.e.m.
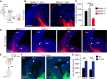
Experimental paradigm used to analyze the involvement of Netrin-1 in thalamic growth.
Wild-type thalamic axons grow significantly less in Netrin-1−/− slices than in control slices (arrowheads). Crystals of DiI (red)-labeled thalamocortical axons, and DAPI nuclear counterstaining is in blue. Scale bar, 300 μm.
Quantification of the data shown in B. **P < 0.01 and ***P < 0.001, Kruskal–Wallis test with Dunn's post hoc analysis. The data are presented as the means ± s.e.m.
Progression of TCA labeled by DiI in the E14.5 thalamus of Dcc+/+ and Dcc−/− brains. The bars indicate the position of the pallial–subpallial boundary for reference, and the arrowheads indicate the delayed position of TCA in the mutants. Scale bar, 200 μm.
Schematic representation of the experimental procedure used to test the effect of increasing the Dcc levels in vivo. A FL-Dcc plasmid was co-electroporated in utero with GFP into the thalamus at E12.5, and the brains were analyzed at E15.5, at the peak of TCA cortical extension.
GFP immunoreactive axons in coronal sections at intermediate cortical levels, with DAPI nuclear counterstaining shown in blue. Overexpression of Dcc led to a significant acceleration in the cortical extension of TCA compared to controls (arrowheads). Scale bar, 300 μm.
Quantification of the data shown in F and data not shown. **P < 0.01, two-tailed Student's t-test. The data are presented as the means ± s.e.m.
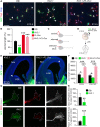
GFP (green), Tuj1 (red), and DAPI (blue) staining in control (Gfp), Kir-transfected, and Kir+FL-Dcc-co-transfected thalamic cells. Increasing the levels of DCC in silenced thalamic neurons rescued the axon growth defect produced by Kir overexpression. The arrowheads indicate the length of the axons. Scale bar, 50 μm.
Quantification of the data shown in A and data not shown. **P < 0.01 and ***P < 0.001; Kruskal–Wallis test with Dunn's post hoc analysis. The data are presented as the means ± s.e.m.
Scheme representing the effect on axon growth after Kir transfection alone or co-transfection with Kir and Kir+FL-Dcc. Red lines represent the status of the spontaneous activity in the cell: no activity, straight line; or spontaneous activity, curved line.
Schematic representation of the experimental procedure used to test the effect of increasing the levels of Dcc in Kir-silenced TCA in vivo. A Kir plasmid was co-electroporated into the thalamus in utero at E12.5 with Gfp or with Gfp plus FL-Dcc, and the brains were analyzed at E15.5.
GFP immunoreactive axons in coronal sections at intermediate cortical levels, with DAPI nuclear counterstaining in blue. Overexpression of Dcc in Kir-silenced axons rescued the cortical extension of TCA silenced axons (arrowheads). Scale bar, 300 μm.
Quantification of the data shown in E and data not shown. *P < 0.05, ***P < 0.001, one-way ANOVA with Tukey's post hoc analysis. The data are presented as the means ± s.e.m.
Expression of DCC (red) and Robo1 (gray) protein levels in E13.5 thalamic growth cones electroporated with Gfp or Kir2.1 plasmids. Scale bar, 5 μm.
Quantification of the data shown in G: **P < 0.01, ***P < 0.001; two-tailed Student's t-test. The data are presented as the means ± s.e.m.

Silencing spontaneous activity induced a 30.38% decrease in expression of the luciferase reporter. *P < 0.05, two-tailed Student's t-test. The data are presented as the means ± s.e.m
Schematic representation of the wild-type and mutated forms of the 1491-bp cloned region of the Dcc promoter.
Significant reduction in the basal transcription of Dcc when NF-κB but not the AP-1 site was mutated (n ≥ 3 replicates). *P < 0.05, Mann–Whitney U-test. The data are presented as the means ± s.e.m.
Luciferase assays to evaluate the contribution of AP-1 and NF-κB sites to the reduction in Dcc by Kir. Mutating only the AP-1 site significantly reduced the dampening of Dcc transcription after the silencing of spontaneous activity (n ≥ 3 replicates). *P < 0.05, Mann–Whitney U-test. The data are presented as the means ± s.e.m.
References
-
- Metin C, Deleglise D, Serafini T, Kennedy TE, Tessier-Lavigne M. A role for netrin-1 in the guidance of cortical efferents. Development. 1997;124:5063–5074. - PubMed
-
- Garel S, Rubenstein JLR. Intermediate targets in formation of topographic projections: inputs from the thalamocortical system. Trends Neurosci. 2004;27:533–539. - PubMed
-
- Mire E, Mezzera C, Leyva-Díaz E, Paternain AV, Squarzoni P, Bluy L, Castillo-Paterna M, López MJ, Peregrín S, Tessier-Lavigne M, et al. Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nat Neurosci. 2012;15:1134–1143. - PubMed
-
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

