Soluble Human Cytomegalovirus gH/gL/pUL128-131 Pentameric Complex, but Not gH/gL, Inhibits Viral Entry to Epithelial Cells and Presents Dominant Native Neutralizing Epitopes
- PMID: 25947373
- PMCID: PMC4481204
- DOI: 10.1074/jbc.M115.652230
Soluble Human Cytomegalovirus gH/gL/pUL128-131 Pentameric Complex, but Not gH/gL, Inhibits Viral Entry to Epithelial Cells and Presents Dominant Native Neutralizing Epitopes
Abstract
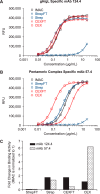
Congenital infection of human cytomegalovirus (HCMV) is one of the leading causes of nongenetic birth defects, and development of a prophylactic vaccine against HCMV is of high priority for public health. The gH/gL/pUL128-131 pentameric complex mediates HCMV entry into endothelial and epithelial cells, and it is a major target for neutralizing antibody responses. To better understand the mechanism by which antibodies interact with the epitopes of the gH/gL/pUL128-131 pentameric complex resulting in viral neutralization, we expressed and purified soluble gH/gL/pUL128-131 pentameric complex and gH/gL from Chinese hamster ovary cells to >95% purity. The soluble gH/gL, which exists predominantly as (gH/gL)2 homodimer with a molecular mass of 220 kDa in solution, has a stoichiometry of 1:1 and a pI of 6.0-6.5. The pentameric complex has a molecular mass of 160 kDa, a stoichiometry of 1:1:1:1:1, and a pI of 7.4-8.1. The soluble pentameric complex, but not gH/gL, adsorbs 76% of neutralizing activities in HCMV human hyperimmune globulin, consistent with earlier reports that the most potent neutralizing epitopes for blocking epithelial infection are unique to the pentameric complex. Functionally, the soluble pentameric complex, but not gH/gL, blocks viral entry to epithelial cells in culture. Our results highlight the importance of the gH/gL/pUL128-131 pentameric complex in HCMV vaccine design and emphasize the necessity to monitor the integrity of the pentameric complex during the vaccine manufacturing process.
Keywords: HCMV; gH/gL/pUL128–131; glycoprotein; neutralizing epitope; pentameric complex; protein complex; protein purification; vaccine; virus entry.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Edward S., Mocarski J., Shenk T., Griffiths P. D., Pass R. F. (2013) Cytomegaloviruses. In Fields Virology (Knipe D. M., Howley P. M., eds) 6th Ed., Lippincott Williams & Wilkins, Philadelphia
-
- Kenneson A., Cannon M. J. (2007) Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17, 253–276 - PubMed
-
- Institute of Medicine CtSPfV (ed) (2001) Vaccines for the 21st century: a tool for decisionmaking, National Academies Press, Washington, D. C. - PubMed
-
- Arvin A. M., Fast P., Myers M., Plotkin S., Rabinovich R., (2004) Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39, 233–239 - PubMed
-
- Sinzger C., Digel M., Jahn G. (2008) Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 325, 63–83 - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

