Dietary n-3 PUFAs Deficiency Increases Vulnerability to Inflammation-Induced Spatial Memory Impairment
- PMID: 25948102
- PMCID: PMC4864653
- DOI: 10.1038/npp.2015.127
Dietary n-3 PUFAs Deficiency Increases Vulnerability to Inflammation-Induced Spatial Memory Impairment
Abstract
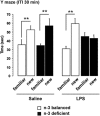
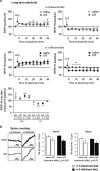
Dietary n-3 polyunsaturated fatty acids (PUFAs) are critical components of inflammatory response and memory impairment. However, the mechanisms underlying the sensitizing effects of low n-3 PUFAs in the brain for the development of memory impairment following inflammation are still poorly understood. In this study, we examined how a 2-month n-3 PUFAs deficiency from pre-puberty to adulthood could increase vulnerability to the effect of inflammatory event on spatial memory in mice. Mice were given diets balanced or deficient in n-3 PUFAs for a 2-month period starting at post-natal day 21, followed by a peripheral administration of lipopolysaccharide (LPS), a bacterial endotoxin, at adulthood. We first showed that spatial memory performance was altered after LPS challenge only in n-3 PUFA-deficient mice that displayed lower n-3/n-6 PUFA ratio in the hippocampus. Importantly, long-term depression (LTD), but not long-term potentiation (LTP) was impaired in the hippocampus of LPS-treated n-3 PUFA-deficient mice. Proinflammatory cytokine levels were increased in the plasma of both n-3 PUFA-deficient and n-3 PUFA-balanced mice. However, only n-3 PUFA-balanced mice showed an increase in cytokine expression in the hippocampus in response to LPS. In addition, n-3 PUFA-deficient mice displayed higher glucocorticoid levels in response to LPS as compared with n-3 PUFA-balanced mice. These results indicate a role for n-3 PUFA imbalance in the sensitization of the hippocampal synaptic plasticity to inflammatory stimuli, which is likely to contribute to spatial memory impairment.
Figures


References
-
- Arsenault D, Julien C, Calon F (2011). Chronic dietary intake of alpha-linolenic acid does not replicate the effects of DHA on passive properties of entorhinal cortex neurons. Br J Nutr 107: 1099–1111. - PubMed
-
- Audoy-Remus J, Bozoyan L, Dumas A, Filali M, Lecours C, Lacroix S et al (2015). GPR84 deficiency reduces microgliosis, but accelerates dendritic degeneration and cognitive decline in a mouse model of Alzheimer's disease. Brain Behav Immun 46: 112–20. - PubMed
-
- Bazinet RP, Laye S (2014). Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15: 771–785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

