Defining the relationship between Plasmodium vivax parasite rate and clinical disease
- PMID: 25948111
- PMCID: PMC4429942
- DOI: 10.1186/s12936-015-0706-3
Defining the relationship between Plasmodium vivax parasite rate and clinical disease
Abstract
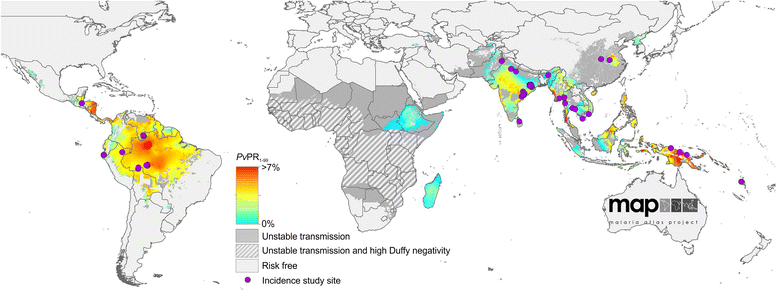
Background: Though essential to the development and evaluation of national malaria control programmes, precise enumeration of the clinical illness burden of malaria in endemic countries remains challenging where local surveillance systems are incomplete. Strategies to infer annual incidence rates from parasite prevalence survey compilations have proven effective in the specific case of Plasmodium falciparum, but have yet to be developed for Plasmodium vivax. Moreover, defining the relationship between P. vivax prevalence and clinical incidence may also allow levels of endemicity to be inferred for areas where the information balance is reversed, that is, incident case numbers are more widely gathered than parasite surveys; both applications ultimately facilitating cartographic estimates of P. vivax transmission intensity and its ensuring disease burden.
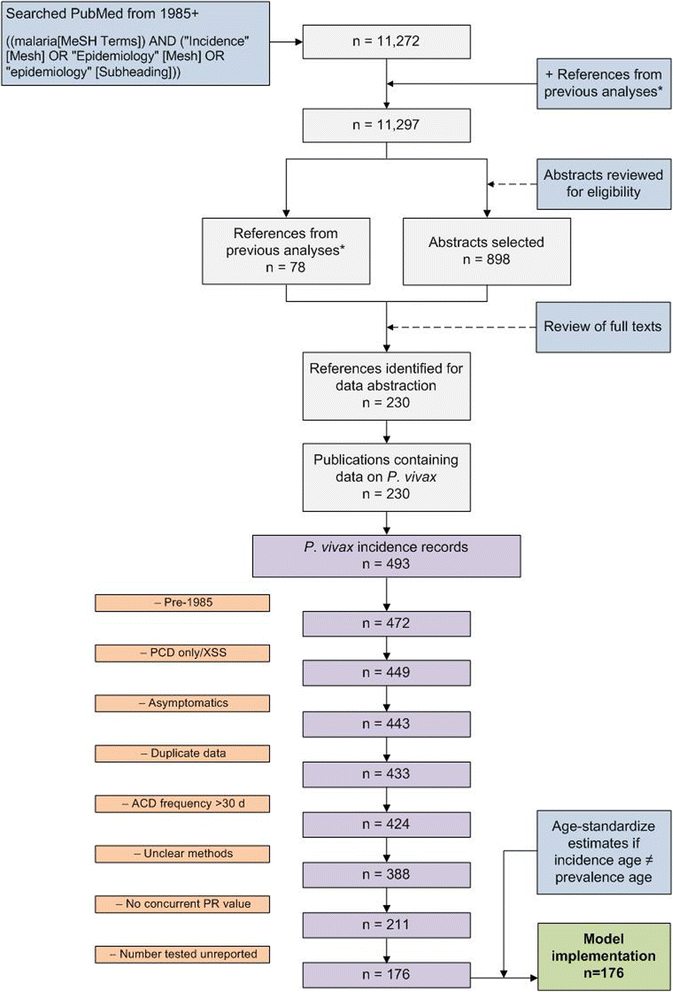
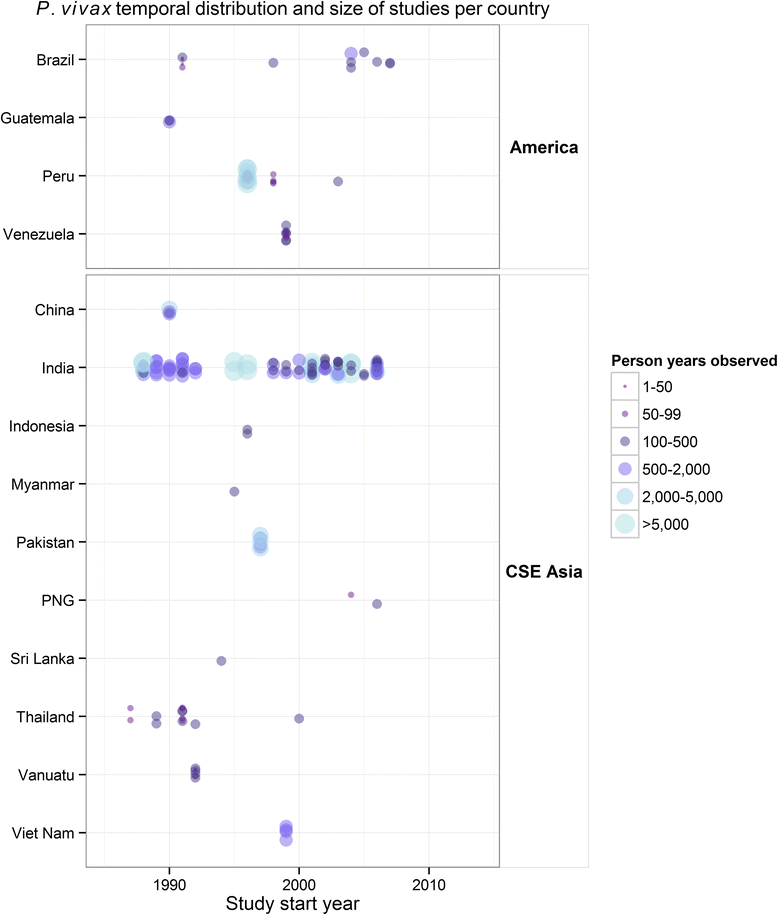
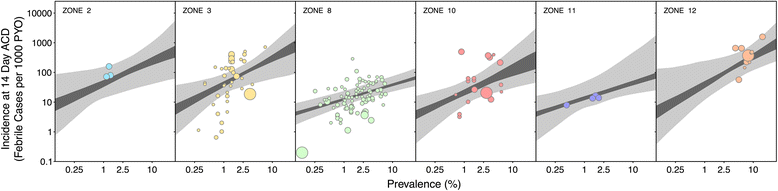
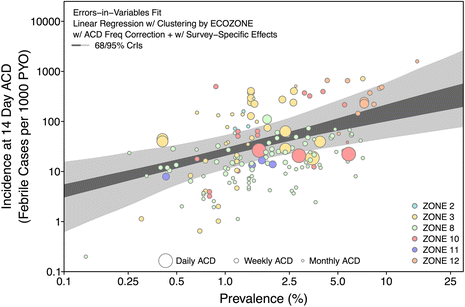
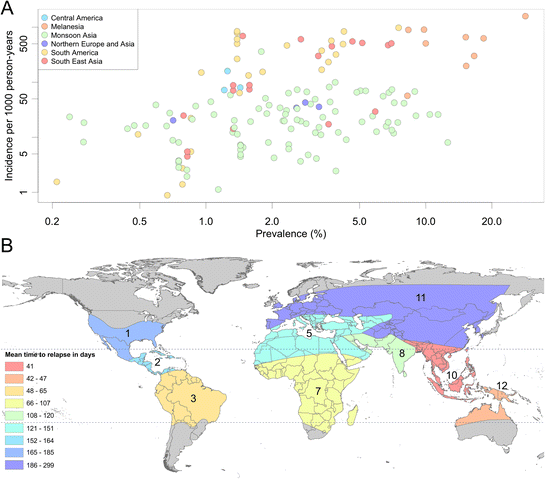
Methods: A search for active case detection surveys was conducted and the recorded incidence values were matched to local, contemporary parasite rate measures and classified to geographic zones of differing relapse phenotypes. A hierarchical Bayesian model was fitted to these data to quantify the relationship between prevalence and incidence while accounting for variation among relapse zones.
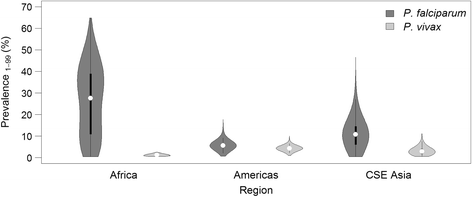
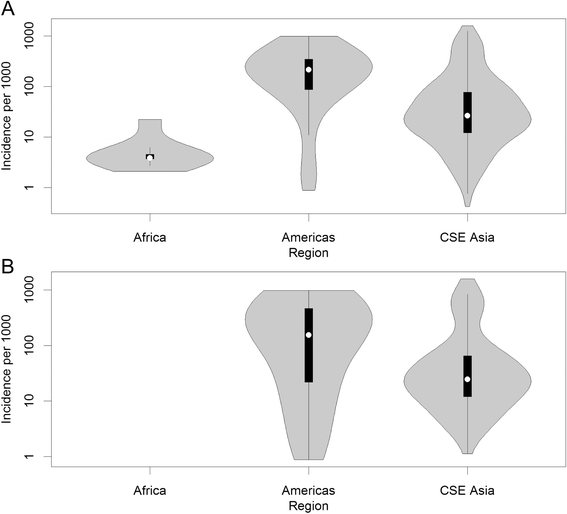
Results: The model, fitted with 176 concurrently measured P. vivax incidence and prevalence records, was a linear regression of the logarithm of incidence against the logarithm of age-standardized prevalence. Specific relationships for the six relapse zones where data were available were drawn, as well as a pooled overall relationship. The slope of the curves varied among relapse zones; zones with short predicted time to relapse had steeper slopes than those observed to contain long-latency relapse phenotypes.
Conclusions: The fitted relationships, along with appropriate uncertainty metrics, allow for estimates of clinical incidence of known confidence to be made from wherever P. vivax prevalence data are available. This is a prerequisite for cartographic-based inferences about the global burden of morbidity due to P. vivax, which will be used to inform control efforts.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

