Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1β Secretion and Photoreceptor Neurodegeneration
- PMID: 25948251
- PMCID: PMC6605270
- DOI: 10.1523/JNEUROSCI.3955-14.2015
Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1β Secretion and Photoreceptor Neurodegeneration
Abstract
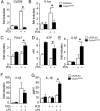
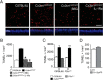
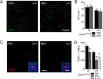
Photoreceptor degeneration in age-related macular degeneration (AMD) is associated with an infiltration and chronic accumulation of mononuclear phagocytes (MPs). We have previously shown that Cx3cr1-deficient mice develop age- and stress- related subretinal accumulation of MPs, which is associated with photoreceptor degeneration. Cx3cr1-deficient MPs have been shown to increase neuronal apoptosis through IL-1β in neuroinflammation of the brain. The reason for increased IL-1β secretion from Cx3cr1-deficient MPs, and whether IL-1β is responsible for increased photoreceptor apoptosis in Cx3cr1-deficient mice, has not been elucidated. Here we show that Cx3cr1-deficient MPs express increased surface P2X7 receptor (P2RX7), which stimulates IL-1β maturation and secretion. P2RX7 and IL-1β inhibition efficiently blunted Cx3cr1-MP-dependent photoreceptor apoptosis in a monocyte/retina coculture system and in light-induced subretinal inflammation of Cx3cr1-deficient mice in vivo. Our results provide an explanation for increased CX3CR1-dependent IL-1β secretion and suggest that IL-1β or P2RX7 inhibition can help inhibit the inflammation-associated photoreceptor cell loss in late AMD, including geographic atrophy, for which no efficient treatment currently exists.
Keywords: IL-1; P2RX7; inflammasome; monocytes; retina.
Copyright © 2015 the authors 0270-6474/15/356987-10$15.00/0.
Figures


Similar articles
-
CD36 Deficiency Inhibits Retinal Inflammation and Retinal Degeneration in Cx3cr1 Knockout Mice.Front Immunol. 2020 Jan 8;10:3032. doi: 10.3389/fimmu.2019.03032. eCollection 2019. Front Immunol. 2020. PMID: 31969887 Free PMC article.
-
CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice.EMBO Mol Med. 2013 Nov;5(11):1775-93. doi: 10.1002/emmm.201302692. Epub 2013 Oct 21. EMBO Mol Med. 2013. PMID: 24142887 Free PMC article.
-
Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration.EMBO Mol Med. 2015 Feb;7(2):211-26. doi: 10.15252/emmm.201404524. EMBO Mol Med. 2015. PMID: 25604058 Free PMC article.
-
APOE Isoforms Control Pathogenic Subretinal Inflammation in Age-Related Macular Degeneration.J Neurosci. 2015 Oct 7;35(40):13568-76. doi: 10.1523/JNEUROSCI.2468-15.2015. J Neurosci. 2015. PMID: 26446211 Free PMC article.
-
On phagocytes and macular degeneration.Prog Retin Eye Res. 2017 Nov;61:98-128. doi: 10.1016/j.preteyeres.2017.06.002. Epub 2017 Jun 7. Prog Retin Eye Res. 2017. PMID: 28602950 Review.
Cited by
-
CX3CR1 deficiency accelerates the development of retinopathy in a rodent model of type 1 diabetes.J Mol Med (Berl). 2016 Nov;94(11):1255-1265. doi: 10.1007/s00109-016-1433-0. Epub 2016 Jun 25. J Mol Med (Berl). 2016. PMID: 27344677 Free PMC article.
-
The Therapeutic Prospects of Targeting IL-1R1 for the Modulation of Neuroinflammation in Central Nervous System Disorders.Int J Mol Sci. 2022 Feb 2;23(3):1731. doi: 10.3390/ijms23031731. Int J Mol Sci. 2022. PMID: 35163653 Free PMC article. Review.
-
IL-33 amplifies an innate immune response in the degenerating retina.J Exp Med. 2016 Feb 8;213(2):189-207. doi: 10.1084/jem.20150894. Epub 2016 Jan 11. J Exp Med. 2016. PMID: 26755704 Free PMC article.
-
MicroRNA-223 Regulates Retinal Function and Inflammation in the Healthy and Degenerating Retina.Front Cell Dev Biol. 2020 Jun 26;8:516. doi: 10.3389/fcell.2020.00516. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32671067 Free PMC article.
-
Plasma and aqueous humor levels of adiponutrin and pannexin 1 in patients with and without diabetic retinopathy.Int J Ophthalmol. 2022 Mar 18;15(3):453-460. doi: 10.18240/ijo.2022.03.13. eCollection 2022. Int J Ophthalmol. 2022. PMID: 35310061 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
