Audio-vocal interaction in single neurons of the monkey ventrolateral prefrontal cortex
- PMID: 25948255
- PMCID: PMC6605268
- DOI: 10.1523/JNEUROSCI.2371-14.2015
Audio-vocal interaction in single neurons of the monkey ventrolateral prefrontal cortex
Abstract
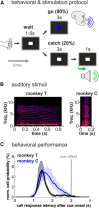
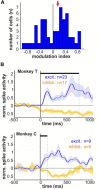
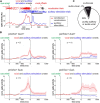
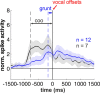
Complex audio-vocal integration systems depend on a strong interconnection between the auditory and the vocal motor system. To gain cognitive control over audio-vocal interaction during vocal motor control, the PFC needs to be involved. Neurons in the ventrolateral PFC (VLPFC) have been shown to separately encode the sensory perceptions and motor production of vocalizations. It is unknown, however, whether single neurons in the PFC reflect audio-vocal interactions. We therefore recorded single-unit activity in the VLPFC of rhesus monkeys (Macaca mulatta) while they produced vocalizations on command or passively listened to monkey calls. We found that 12% of randomly selected neurons in VLPFC modulated their discharge rate in response to acoustic stimulation with species-specific calls. Almost three-fourths of these auditory neurons showed an additional modulation of their discharge rates either before and/or during the monkeys' motor production of vocalization. Based on these audio-vocal interactions, the VLPFC might be well positioned to combine higher order auditory processing with cognitive control of the vocal motor output. Such audio-vocal integration processes in the VLPFC might constitute a precursor for the evolution of complex learned audio-vocal integration systems, ultimately giving rise to human speech.
Keywords: audio-vocal integration; cognitive control; monkey homolog of human Broca's area; prefrontal cortex; vocal motor control; vocalization.
Copyright © 2015 the authors 0270-6474/15/357030-11$15.00/0.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
