Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system
- PMID: 25948256
- PMCID: PMC6605260
- DOI: 10.1523/JNEUROSCI.5128-14.2015
Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system
Abstract
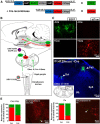
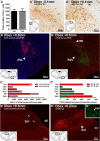
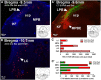
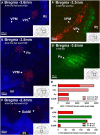
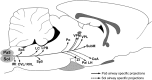
Sensory nerves innervating the mucosa of the airways monitor the local environment for the presence of irritant stimuli and, when activated, provide input to the nucleus of the solitary tract (Sol) and paratrigeminal nucleus (Pa5) in the medulla to drive a variety of protective behaviors. Accompanying these behaviors are perceivable sensations that, particularly for stimuli in the proximal end of the airways, can be discrete and localizable. Airway sensations likely reflect the ascending airway sensory circuitry relayed via the Sol and Pa5, which terminates broadly throughout the CNS. However, the relative contribution of the Sol and Pa5 to these ascending pathways is not known. In the present study, we developed and characterized a novel conditional anterograde transneuronal viral tracing system based on the H129 strain of herpes simplex virus 1 and used this system in rats along with conventional neuroanatomical tracing with cholera toxin B to identify subcircuits in the brainstem and forebrain that are in receipt of relayed airway sensory inputs via the Sol and Pa5. We show that both the Pa5 and proximal airways disproportionately receive afferent terminals arising from the jugular (rather than nodose) vagal ganglia and the output of the Pa5 is predominately directed toward the ventrobasal thalamus. We propose the existence of a somatosensory-like pathway from the proximal airways involving jugular ganglia afferents, the Pa5, and the somatosensory thalamus and suggest that this pathway forms the anatomical framework for sensations arising from the proximal airway mucosa.
Keywords: HSV-1 H129; airway sensations; neuroanatomical tracing; nucleus of the solitary tract; paratrigeminal; visceral sensation.
Copyright © 2015 the authors 0270-6474/15/357041-15$15.00/0.
Figures


References
-
- Baker CV, Schlosser G. The evolutionary origin of neural crest and placodes. J Exp Zool B Mol Dev Evol. 2005;304:269–273. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
