Neuronal morphology generates high-frequency firing resonance
- PMID: 25948257
- PMCID: PMC6605263
- DOI: 10.1523/JNEUROSCI.3924-14.2015
Neuronal morphology generates high-frequency firing resonance
Abstract
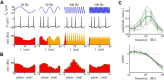
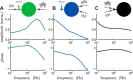
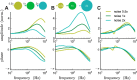
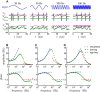
The attenuation of neuronal voltage responses to high-frequency current inputs by the membrane capacitance is believed to limit single-cell bandwidth. However, neuronal populations subject to stochastic fluctuations can follow inputs beyond this limit. We investigated this apparent paradox theoretically and experimentally using Purkinje cells in the cerebellum, a motor structure that benefits from rapid information transfer. We analyzed the modulation of firing in response to the somatic injection of sinusoidal currents. Computational modeling suggested that, instead of decreasing with frequency, modulation amplitude can increase up to high frequencies because of cellular morphology. Electrophysiological measurements in adult rat slices confirmed this prediction and displayed a marked resonance at 200 Hz. We elucidated the underlying mechanism, showing that the two-compartment morphology of the Purkinje cell, interacting with a simple spiking mechanism and dendritic fluctuations, is sufficient to create high-frequency signal amplification. This mechanism, which we term morphology-induced resonance, is selective for somatic inputs, which in the Purkinje cell are exclusively inhibitory. The resonance sensitizes Purkinje cells in the frequency range of population oscillations observed in vivo.
Keywords: Purkinje cell; computational modelling; information bandwidth; neural dynamics.
Copyright © 2015 the authors 0270-6474/15/357056-13$15.00/0.
Figures



Similar articles
-
Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs.J Physiol. 2006 Jul 15;574(Pt 2):491-507. doi: 10.1113/jphysiol.2006.108282. Epub 2006 May 18. J Physiol. 2006. PMID: 16709640 Free PMC article.
-
Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum.J Physiol. 1986 Feb;371:89-114. doi: 10.1113/jphysiol.1986.sp015963. J Physiol. 1986. PMID: 3701658 Free PMC article.
-
Firing dynamics of cerebellar purkinje cells.J Neurophysiol. 2007 Jul;98(1):278-94. doi: 10.1152/jn.00306.2007. Epub 2007 May 9. J Neurophysiol. 2007. PMID: 17493923
-
Climbing fibers mediate vestibular modulation of both "complex" and "simple spikes" in Purkinje cells.Cerebellum. 2015 Oct;14(5):597-612. doi: 10.1007/s12311-015-0725-1. Cerebellum. 2015. PMID: 26424151 Review.
-
The beat goes on: spontaneous firing in mammalian neuronal microcircuits.J Neurosci. 2004 Oct 20;24(42):9215-9. doi: 10.1523/JNEUROSCI.3375-04.2004. J Neurosci. 2004. PMID: 15496653 Free PMC article. Review. No abstract available.
Cited by
-
Differential polarization of cortical pyramidal neuron dendrites through weak extracellular fields.PLoS Comput Biol. 2018 May 4;14(5):e1006124. doi: 10.1371/journal.pcbi.1006124. eCollection 2018 May. PLoS Comput Biol. 2018. PMID: 29727454 Free PMC article.
-
Coding Capacity of Purkinje Cells With Different Schemes of Morphological Reduction.Front Comput Neurosci. 2019 May 15;13:29. doi: 10.3389/fncom.2019.00029. eCollection 2019. Front Comput Neurosci. 2019. PMID: 31156415 Free PMC article.
-
From Neuron Biophysics to Orientation Selectivity in Electrically Coupled Networks of Neocortical L2/3 Large Basket Cells.Cereb Cortex. 2016 Aug;26(8):3655-3668. doi: 10.1093/cercor/bhw166. Epub 2016 Jun 9. Cereb Cortex. 2016. PMID: 27288316 Free PMC article.
-
Impact of Purkinje Cell Simple Spike Synchrony on Signal Transmission from Flocculus.Cerebellum. 2022 Dec;21(6):879-904. doi: 10.1007/s12311-021-01332-w. Epub 2021 Oct 19. Cerebellum. 2022. PMID: 34665396
-
Strings on a Violin: Location Dependence of Frequency Tuning in Active Dendrites.Front Cell Neurosci. 2017 Mar 13;11:72. doi: 10.3389/fncel.2017.00072. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28348519 Free PMC article. Review.
References
-
- Adrian ED. Discharge frequencies in the cerebral and cerebellar cortex. Proc Phys Soc. 1935;83:32–33.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
