Unilateral prefrontal lesions impair memory-guided comparisons of contralateral visual motion
- PMID: 25948260
- PMCID: PMC4420779
- DOI: 10.1523/JNEUROSCI.5265-14.2015
Unilateral prefrontal lesions impair memory-guided comparisons of contralateral visual motion
Abstract
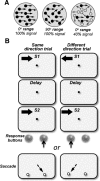
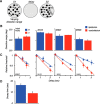
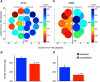
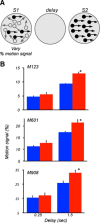
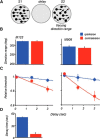
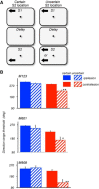
The contribution of the lateral prefrontal cortex (LPFC) to working memory is the topic of active debate. On the one hand, it has been argued that the persistent delay activity in LPFC recorded during some working memory tasks is a reflection of sensory storage, the notion supported by some lesion studies. On the other hand, there is emerging evidence that the LPFC plays a key role in the maintenance of sensory information not by storing relevant visual signals but by allocating visual attention to such stimuli. In this study, we addressed this question by examining the effects of unilateral LPFC lesions during a working memory task requiring monkeys to compare directions of two moving stimuli, separated by a delay. The lesions resulted in impaired thresholds for contralesional stimuli at longer delays, and these deficits were most dramatic when the task required rapid reallocation of spatial attention. In addition, these effects were equally pronounced when the remembered stimuli were at threshold or moved coherently. The contralesional nature of the deficits points to the importance of the interactions between the LPFC and the motion processing neurons residing in extrastriate area MT. Delay-specificity of the deficit supports LPFC involvement in the maintenance stage of the comparison task. However, because this deficit was independent of stimulus features giving rise to the remembered direction and was most pronounced during rapid shifts of attention, its role is more likely to be attending and accessing the preserved motion signals rather than their storage.
Keywords: cortical damage; direction discrimination; motion perception; prefrontal cortex; visual attention; working memory.
Copyright © 2015 the authors 0270-6474/15/357095-11$15.00/0.
Figures

Similar articles
-
Prefrontal Neurons Represent Motion Signals from Across the Visual Field But for Memory-Guided Comparisons Depend on Neurons Providing These Signals.J Neurosci. 2016 Sep 7;36(36):9351-64. doi: 10.1523/JNEUROSCI.0843-16.2016. J Neurosci. 2016. PMID: 27605611 Free PMC article.
-
Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task.J Neurosci. 2006 Nov 8;26(45):11726-42. doi: 10.1523/JNEUROSCI.3420-06.2006. J Neurosci. 2006. PMID: 17093094 Free PMC article.
-
Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits.J Neurosci. 2016 Jan 13;36(2):489-505. doi: 10.1523/JNEUROSCI.3678-15.2016. J Neurosci. 2016. PMID: 26758840 Free PMC article.
-
Linking Neuronal Direction Selectivity to Perceptual Decisions About Visual Motion.Annu Rev Vis Sci. 2020 Sep 15;6:335-362. doi: 10.1146/annurev-vision-121219-081816. Annu Rev Vis Sci. 2020. PMID: 32936737 Review.
-
Neural mechanisms of information storage in visual short-term memory.Vision Res. 2016 Nov;128:53-67. doi: 10.1016/j.visres.2016.09.010. Epub 2016 Oct 4. Vision Res. 2016. PMID: 27668990 Free PMC article. Review.
Cited by
-
A Normalization Circuit Underlying Coding of Spatial Attention in Primate Lateral Prefrontal Cortex.eNeuro. 2019 Apr 15;6(2):ENEURO.0301-18.2019. doi: 10.1523/ENEURO.0301-18.2019. eCollection 2019 Mar-Apr. eNeuro. 2019. PMID: 31001577 Free PMC article.
-
Persistent Spiking Activity Underlies Working Memory.J Neurosci. 2018 Aug 8;38(32):7020-7028. doi: 10.1523/JNEUROSCI.2486-17.2018. J Neurosci. 2018. PMID: 30089641 Free PMC article.
-
Prefrontal Neurons Represent Motion Signals from Across the Visual Field But for Memory-Guided Comparisons Depend on Neurons Providing These Signals.J Neurosci. 2016 Sep 7;36(36):9351-64. doi: 10.1523/JNEUROSCI.0843-16.2016. J Neurosci. 2016. PMID: 27605611 Free PMC article.
-
Interhemispheric transfer of working memories.Neuron. 2021 Mar 17;109(6):1055-1066.e4. doi: 10.1016/j.neuron.2021.01.016. Epub 2021 Feb 8. Neuron. 2021. PMID: 33561399 Free PMC article.
-
Top-Down Control of Visual Attention by the Prefrontal Cortex. Functional Specialization and Long-Range Interactions.Front Neurosci. 2017 Sep 29;11:545. doi: 10.3389/fnins.2017.00545. eCollection 2017. Front Neurosci. 2017. PMID: 29033784 Free PMC article. Review.
References
-
- Bisley JW, Zaksas D, Pasternak T. Microstimulation of cortical area MT affects performance on a visual working memory task. J Neurophysiol. 2001;85:187–196. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
