GIRK Channels Modulate Opioid-Induced Motor Activity in a Cell Type- and Subunit-Dependent Manner
- PMID: 25948263
- PMCID: PMC4420781
- DOI: 10.1523/JNEUROSCI.5051-14.2015
GIRK Channels Modulate Opioid-Induced Motor Activity in a Cell Type- and Subunit-Dependent Manner
Abstract
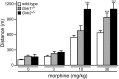
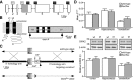
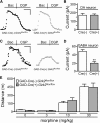
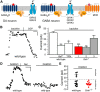
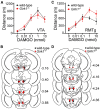
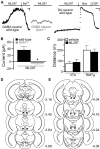
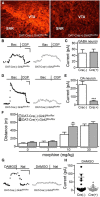
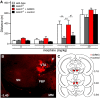
G-protein-gated inwardly rectifying K(+) (GIRK/Kir3) channel activation underlies key physiological effects of opioids, including analgesia and dependence. GIRK channel activation has also been implicated in the opioid-induced inhibition of midbrain GABA neurons and consequent disinhibition of dopamine (DA) neurons in the ventral tegmental area (VTA). Drug-induced disinhibition of VTA DA neurons has been linked to reward-related behaviors and underlies opioid-induced motor activation. Here, we demonstrate that mouse VTA GABA neurons express a GIRK channel formed by GIRK1 and GIRK2 subunits. Nevertheless, neither constitutive genetic ablation of Girk1 or Girk2, nor the selective ablation of GIRK channels in GABA neurons, diminished morphine-induced motor activity in mice. Moreover, direct activation of GIRK channels in midbrain GABA neurons did not enhance motor activity. In contrast, genetic manipulations that selectively enhanced or suppressed GIRK channel function in midbrain DA neurons correlated with decreased and increased sensitivity, respectively, to the motor-stimulatory effect of systemic morphine. Collectively, these data support the contention that the unique GIRK channel subtype in VTA DA neurons, the GIRK2/GIRK3 heteromer, regulates the sensitivity of the mouse mesolimbic DA system to drugs with addictive potential.
Keywords: GIRK; Kir3; conditional knockout; morphine; rostromedial tegmental area; ventral tegmental area.
Copyright © 2015 the authors 0270-6474/15/357131-12$15.00/0.
Figures
References
-
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Luján R, Wickman K. Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci. 2011;31:12251–12257. doi: 10.1523/JNEUROSCI.0494-11.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DA029343/DA/NIDA NIH HHS/United States
- DA011806/DA/NIDA NIH HHS/United States
- MH061933/MH/NIMH NIH HHS/United States
- T32 DA007097/DA/NIDA NIH HHS/United States
- R01 DA037170/DA/NIDA NIH HHS/United States
- DA007097/DA/NIDA NIH HHS/United States
- T32 DA007234/DA/NIDA NIH HHS/United States
- DA029343/DA/NIDA NIH HHS/United States
- R01 DA034696/DA/NIDA NIH HHS/United States
- P50 DA011806/DA/NIDA NIH HHS/United States
- R01 MH061933/MH/NIMH NIH HHS/United States
- DA007234/DA/NIDA NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 GM008471/GM/NIGMS NIH HHS/United States
- GM008471/GM/NIGMS NIH HHS/United States
- DA07234/DA/NIDA NIH HHS/United States
- CA068485/CA/NCI NIH HHS/United States
- R01 AA018734/AA/NIAAA NIH HHS/United States
- DA034696/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
