Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability
- PMID: 25948275
- PMCID: PMC4420788
- DOI: 10.1523/JNEUROSCI.4747-14.2015
Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability
Abstract
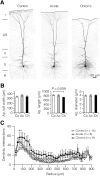
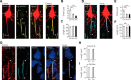
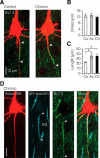
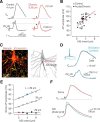
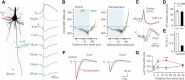
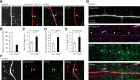
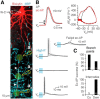
Myelination and voltage-gated ion channel clustering at the nodes of Ranvier are essential for the rapid saltatory conduction of action potentials. Whether myelination influences the structural organization of the axon initial segment (AIS) and action potential initiation is poorly understood. Using the cuprizone mouse model, we combined electrophysiological recordings with immunofluorescence of the voltage-gated Nav1.6 and Kv7.3 subunits and anchoring proteins to analyze the functional and structural properties of single demyelinated neocortical L5 axons. Whole-cell recordings demonstrated that neurons with demyelinated axons were intrinsically more excitable, characterized by increased spontaneous suprathreshold depolarizations as well as antidromically propagating action potentials ectopically generated in distal parts of the axon. Immunofluorescence examination of demyelinated axons showed that βIV-spectrin, Nav1.6, and the Kv7.3 channels in nodes of Ranvier either dissolved or extended into the paranodal domains. In contrast, while the AIS in demyelinated axons started more closely to the soma, ankyrin G, βIV-spectrin, and the ion channel expression were maintained. Structure-function analysis and computational modeling, constrained by the AIS location and realistic dendritic and axonal morphologies, confirmed that a more proximal onset of the AIS slightly reduced the efficacy of action potential generation, suggesting a compensatory role. These results suggest that oligodendroglial myelination is not only important for maximizing conduction velocity, but also for limiting hyperexcitability of pyramidal neurons.
Keywords: Kv7.3; Nav1.6; axon; axon initial segment; demyelination; node of Ranvier.
Copyright © 2015 Hamada and Kole.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
