Orexin Signaling in the VTA Gates Morphine-Induced Synaptic Plasticity
- PMID: 25948277
- PMCID: PMC6605267
- DOI: 10.1523/JNEUROSCI.4385-14.2015
Orexin Signaling in the VTA Gates Morphine-Induced Synaptic Plasticity
Abstract
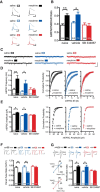
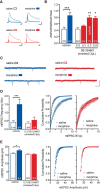
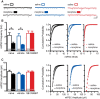
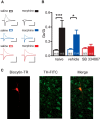
Dopamine neurons in the ventral tegmental area (VTA) are a key target of addictive drugs, and neuroplasticity in this region may underlie some of the core features of addiction. From the very first exposure, all drugs of abuse induce synaptic plasticity in the VTA. However, it is not well understood how this diverse group of drugs brings about common synaptic change. Orexin (also known as hypocretin) is a lateral hypothalamic neuropeptide released into the VTA that promotes drug-seeking behaviors and potentiates excitatory synaptic transmission onto VTA dopamine neurons. Here we show that signaling at orexin receptor type 1 (OxR1) in the VTA is required for morphine-induced plasticity of dopamine neurons. Systemic or intra-VTA administration of the OxR1 antagonist SB 334867 in rats blocked a morphine-induced increase in the AMPAR/NMDAR ratio, an increase in presynaptic glutamate release, and a postsynaptic change in AMPAR number or function, including a switch in subunit composition. Furthermore, SB 334867 blocked a morphine-induced decrease in presynaptic GABA release, and a morphine-induced shift in the balance of excitatory and inhibitory synaptic inputs to dopamine neurons. These findings identify a novel role for orexin in morphine-induced plasticity in the VTA and provide a mechanism by which orexin can gate the output of dopamine neurons.
Keywords: AMPA; NMDA; dopamine; morphine; orexin; ventral tegmental area.
Copyright © 2015 the authors 0270-6474/15/357295-09$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
