Endothelial progenitor cells inhibit platelet function in a P-selectin-dependent manner
- PMID: 25948279
- PMCID: PMC4438565
- DOI: 10.1186/s12967-015-0508-y
Endothelial progenitor cells inhibit platelet function in a P-selectin-dependent manner
Abstract
Background: The role of endothelial progenitor cells (EPCs) in vascular repair is related to their recruitment at the sites of injury and their interaction with different components of the circulatory system. We have previously shown that EPCs bind and inhibit platelet function and impair thrombus formation via prostacyclin secretion, but the role of EPC binding to platelet P-selectin in this process has not been fully characterized. In the present study, we assessed the impact of EPCs on thrombus formation and we addressed the implication of P-selectin in this process.
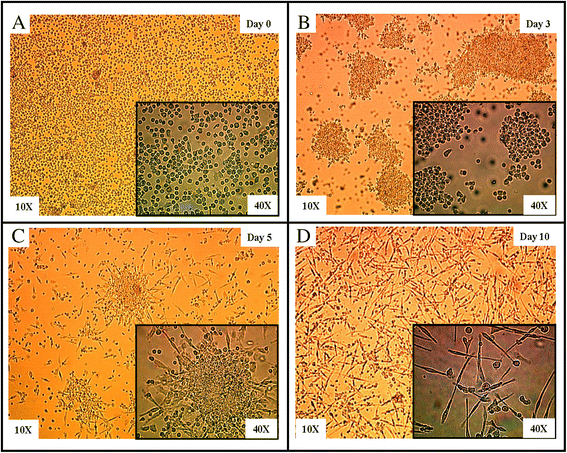
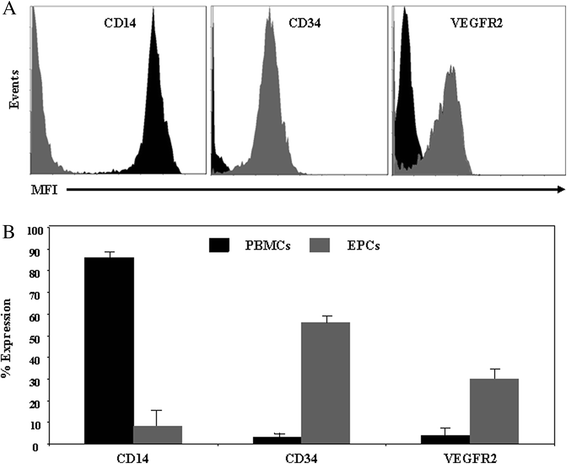
Methods: EPCs were generated from human peripheral blood mononuclear cells cultured on fibronectin in conditioned media. The impact of EPCs on platelet aggregation and thrombus formation was investigated in P-selectin deficient (P-sel(-/-)) mice and their wild-type (WT) counterparts.
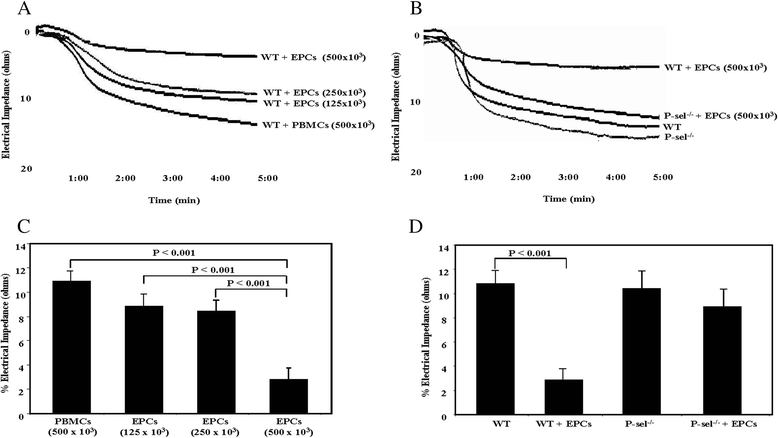
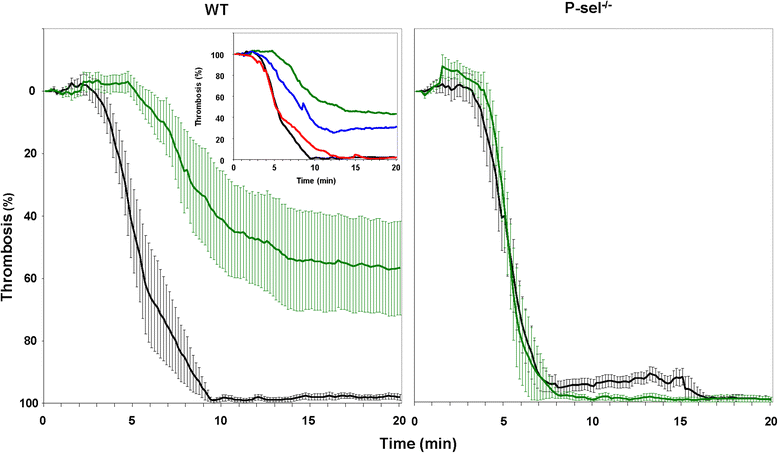
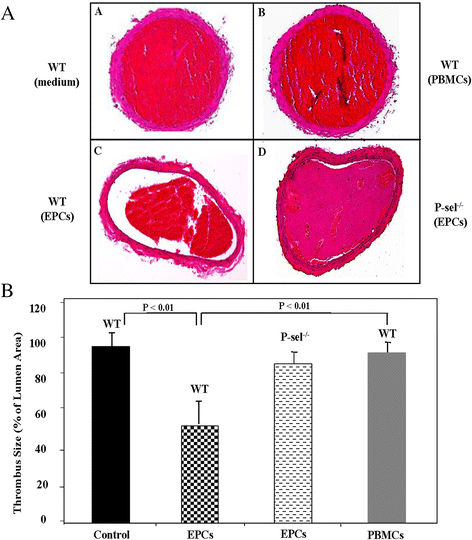
Results: EPCs significantly and dose-dependently impaired collagen-induced whole blood platelet aggregation in WT mice, whereas no effects were observed in P-sel(-/-) mice. Moreover, in a ferric chloride-induced arterial thrombosis model, infusion of EPCs significantly reduced thrombus formation in WT, but not in P-sel(-/-) mice. Furthermore, the relative mass of thrombi generated in EPC-treated P-sel(-/-) mice were significantly larger than those in EPC-treated WT mice, and the number of EPCs recruited within the thrombi and along the arterial wall was reduced in P-sel(-/-) mice as compared to WT mice.
Conclusion: This study shows that EPCs impair platelet aggregation and reduce thrombus formation via a cellular mechanism involving binding to platelet P-selectin. These findings add new insights into the role of EPC-platelet interactions in the regulation of thrombotic events during vascular repair.
Figures

Similar articles
-
Early outgrowth cells versus endothelial colony forming cells functions in platelet aggregation.J Transl Med. 2015 Nov 9;13:353. doi: 10.1186/s12967-015-0723-6. J Transl Med. 2015. PMID: 26552480 Free PMC article.
-
Endothelial progenitor cells bind and inhibit platelet function and thrombus formation.Circulation. 2009 Dec 1;120(22):2230-9. doi: 10.1161/CIRCULATIONAHA.109.894642. Epub 2009 Nov 16. Circulation. 2009. PMID: 19917882 Free PMC article.
-
Platelet P-selectin plays an important role in arterial thrombogenesis by forming large stable platelet-leukocyte aggregates.J Am Coll Cardiol. 2005 Apr 19;45(8):1280-6. doi: 10.1016/j.jacc.2004.12.071. J Am Coll Cardiol. 2005. PMID: 15837262
-
P-selectin in arterial thrombosis.Z Kardiol. 2004 Nov;93(11):855-63. doi: 10.1007/s00392-004-0146-5. Z Kardiol. 2004. PMID: 15568145 Review.
-
Effects of shear stress on endothelial progenitor cells.J Biomed Nanotechnol. 2014 Oct;10(10):2586-97. doi: 10.1166/jbn.2014.2014. J Biomed Nanotechnol. 2014. PMID: 25992410 Review.
Cited by
-
Early outgrowth cells versus endothelial colony forming cells functions in platelet aggregation.J Transl Med. 2015 Nov 9;13:353. doi: 10.1186/s12967-015-0723-6. J Transl Med. 2015. PMID: 26552480 Free PMC article.
-
Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73-converted adenosine.Stem Cell Res Ther. 2018 Jul 4;9(1):184. doi: 10.1186/s13287-018-0936-8. Stem Cell Res Ther. 2018. PMID: 29973267 Free PMC article.
-
Statins and Hemostasis: Therapeutic Potential Based on Clinical Evidence.Adv Exp Med Biol. 2023;1408:25-47. doi: 10.1007/978-3-031-26163-3_2. Adv Exp Med Biol. 2023. PMID: 37093420 Review.
-
The Potential Role of Connexins in the Pathogenesis of Atherosclerosis.Int J Mol Sci. 2023 Jan 30;24(3):2600. doi: 10.3390/ijms24032600. Int J Mol Sci. 2023. PMID: 36768920 Free PMC article. Review.
References
-
- Kong D, Melo LG, Gnecchi M, Zhang L, Mostoslavsky G, Liew CC, et al. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation. 2004;110(14):2039–46. doi: 10.1161/01.CIR.0000143161.01901.BD. - DOI - PubMed
-
- Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108(21):2710–5. doi: 10.1161/01.CIR.0000096490.16596.A6. - DOI - PubMed
-
- Larsen K, Cheng C, Tempel D, Parker S, Yazdani S, den Dekker WK, et al. Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur Heart J. 2012;33(1):120–8. doi: 10.1093/eurheartj/ehr196. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

