Fibroblast growth factor-2 regulates human cardiac myofibroblast-mediated extracellular matrix remodeling
- PMID: 25948488
- PMCID: PMC4438633
- DOI: 10.1186/s12967-015-0510-4
Fibroblast growth factor-2 regulates human cardiac myofibroblast-mediated extracellular matrix remodeling
Abstract
Background: Tissue fibrosis and chamber remodeling is a hallmark of the failing heart and the final common pathway for heart failure of diverse etiologies. Sustained elevation of pro-fibrotic cytokine transforming growth factor-beta1 (TGFβ1) induces cardiac myofibroblast-mediated fibrosis and progressive structural tissue remodeling.
Objectives: We examined the effects of low molecular weight fibroblast growth factor (LMW-FGF-2) on human cardiac myofibroblast-mediated extracellular matrix (ECM) dysregulation and remodeling.
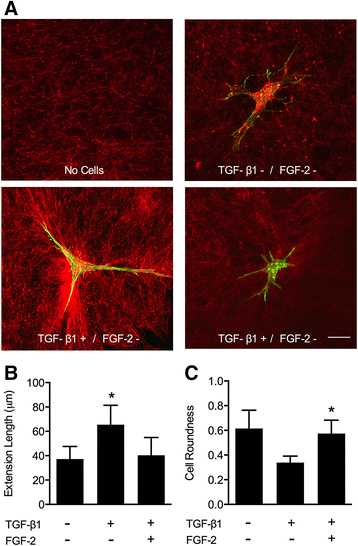
Methods: Human cardiac biopsies were obtained during open-heart surgery and myofibroblasts were isolated, passaged, and seeded within type I collagen matrices. To induce myofibroblast activation and ECM remodeling, myofibroblast-seeded collagen gels were exposed to TGFβ1. The extent of ECM contraction, myofibroblast activation, ECM dysregulation, and cell apoptosis was determined in the presence of LMW-FGF-2 and compared to its absence. Using a novel floating nylon-grid supported thin collagen gel culture platform system, myofibroblast activation and local ECM remodeling around isolated single cells was imaged using confocal microscopy and quantified by image analysis.
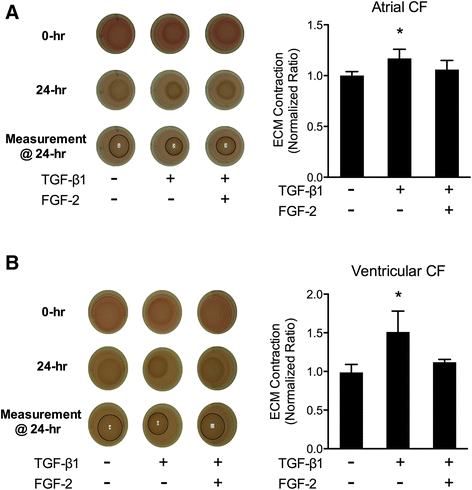
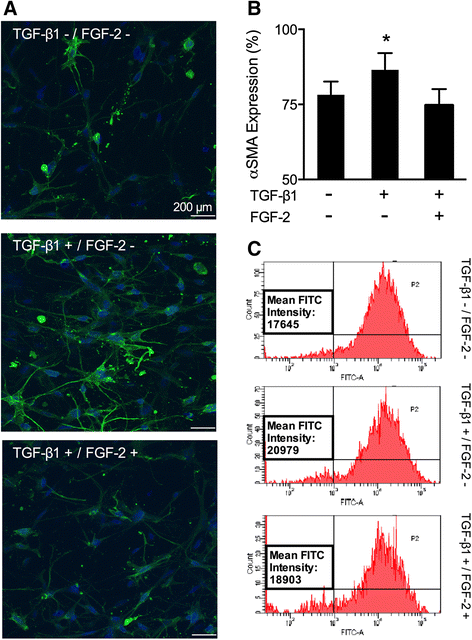
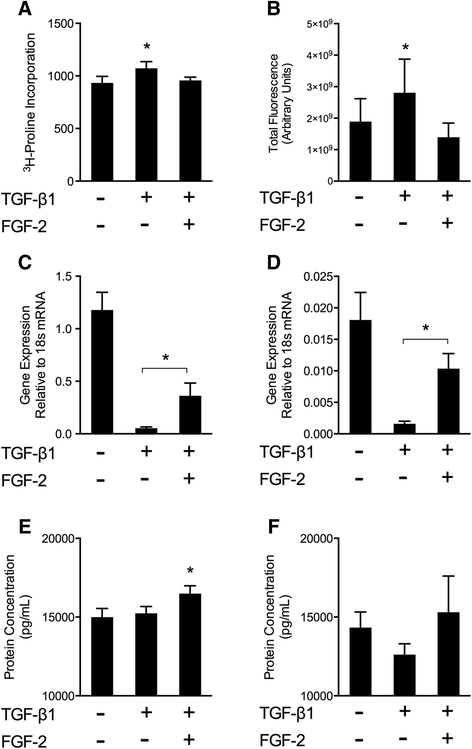
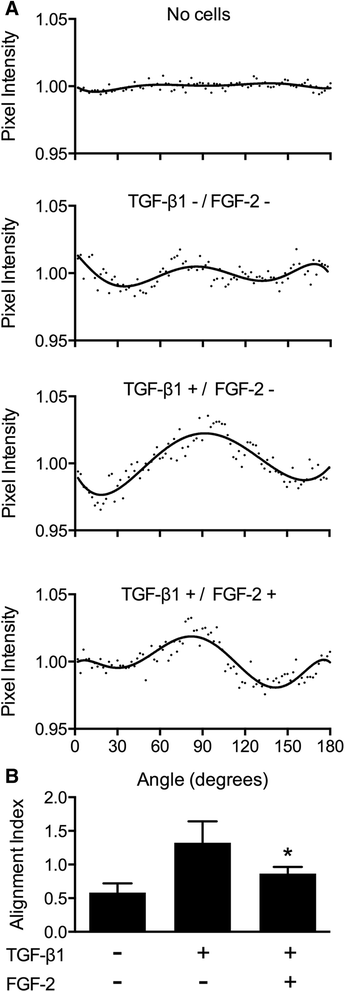
Results: TGFβ1 induced significant myofibroblast activation and ECM dysregulation as evidenced by collagen gel contraction, structural ECM remodeling, collagen synthesis, ECM degradation, and altered TIMP expression. LMW-FGF-2 significantly attenuated TGFβ1 induced myofibroblast-mediated ECM remodeling. These observations were similar using either ventricular or atrial-derived cardiac myofibroblasts. In addition, for the first time using individual cells, LMW-FGF-2 was observed to attenuate cardiac myofibroblast activation and prevent local cell-mediated ECM perturbations.
Conclusions: LMW-FGF-2 attenuates human cardiac myofibroblast-mediated ECM remodeling and may prevent progressive maladaptive chamber remodeling and tissue fibrosis for patients with diverse structural heart diseases.
Figures
References
-
- Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. CircRes. 2002;90(5):520–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

