Tumor Necrosis Factor-α Underlies Loss of Cortical Dendritic Spine Density in a Mouse Model of Congestive Heart Failure
- PMID: 25948533
- PMCID: PMC4599420
- DOI: 10.1161/JAHA.115.001920
Tumor Necrosis Factor-α Underlies Loss of Cortical Dendritic Spine Density in a Mouse Model of Congestive Heart Failure
Abstract
Background: Heart failure (HF) is a progressive disorder characterized by reduced cardiac output and increased peripheral resistance, ultimately leading to tissue perfusion deficits and devastating consequences for several organs including the brain. We previously described a tumor necrosis factor-α (TNF-α)-dependent enhancement of posterior cerebral artery tone and concomitant reduced cerebral blood flow in a mouse model of early HF in which blood pressure remains minimally affected. HF is often associated with cognitive impairments such as memory deficits, even before any overt changes in brain structure and function occur. The pathophysiology underlying the development of cognitive impairments in HF is unknown, and appropriate treatment strategies are lacking.
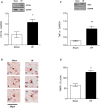
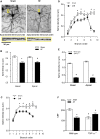
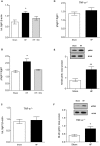
Methods and results: We used a well-established mouse model in which HF was induced by experimental myocardial infarction produced by permanent surgical ligation of the left anterior descending coronary artery (infarct size ≈25% of the left ventricular wall). Ligated mice developed enlarged hearts, congested lungs, and reduced cardiac output and blood pressure, with elevated peripheral resistance within 6 to 8 weeks after ligation. In this study, we demonstrated the significance of the proinflammatory cytokine TNF-α during HF-mediated neuroinflammation and associated impaired hippocampus-independent nonspatial episodic memory function. Augmented cerebral TNF-α expression and microglial activation in HF mice, indicative of brain inflammation, were accompanied by morphological changes and significant reduction of cortical dendritic spines (61.39±8.61% for basal and 61.04±9.18% for apical spines [P<0.001]). The significance of TNF-α signaling during the observed HF-mediated neurodegenerative processes is supported by evidence showing that sequestration or genetic deletion of TNF-α ameliorates the observed reduction of cortical dendritic spines (33.51±7.63% for basal and 30.13±6.98% for apical spines in wild-type mice treated with etanercept; 17.09±6.81% for basal and 17.21±7.29% for apical spines in TNF-α(-/-)). Moreover, our data suggest that alterations in cerebral serum and glucocorticoid-inducible kinase 1 (SgK1) expression and phosphorylation during HF may be TNF-α dependent and that an increase of SgK1 phosphorylation potentially plays a role in the HF-associated reduction of dendritic spine density.
Conclusions: Our findings demonstrate that TNF-α plays a pivotal role in HF-mediated neuroinflammation and associated alterations of cortical dendritic spine density and has the potential to reveal novel treatment strategies for HF-associated memory deficits.
Keywords: heart failure; inflammation; nervous system.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures



References
-
- Havranek EP, Dwinnell B, Smith KS. Multiorgan failure after cardiac arrest in a 20-year-old man. Circulation. 1995;92:3139–3143. - PubMed
-
- Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Chui M, Deer M, Murray MD. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52:207–216. - PubMed
-
- Vogels RL, Oosterman JM, van Harten B, Gouw AA, Schroeder-Tanka JM, Scheltens P, van der Flier WM, Weinstein HC. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;24:418–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

