LMP1-Induced Sumoylation Influences the Maintenance of Epstein-Barr Virus Latency through KAP1
- PMID: 25948750
- PMCID: PMC4505653
- DOI: 10.1128/JVI.00711-15
LMP1-Induced Sumoylation Influences the Maintenance of Epstein-Barr Virus Latency through KAP1
Abstract
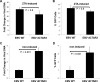
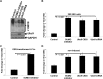
As a herpesvirus, Epstein-Barr virus (EBV) establishes a latent infection that can periodically undergo reactivation, resulting in lytic replication and the production of new infectious virus. Latent membrane protein-1 (LMP1), the principal viral oncoprotein, is a latency-associated protein implicated in regulating viral reactivation and the maintenance of latency. We recently found that LMP1 hijacks the SUMO-conjugating enzyme Ubc9 via its C-terminal activating region-3 (CTAR3) and induces the sumoylation of cellular proteins. Because protein sumoylation can promote transcriptional repression, we hypothesized that LMP1-induced protein sumoylation induces the repression of EBV lytic promoters and helps maintain the viral genome in its latent state. We now show that with inhibition of LMP1-induced protein sumoylation, the latent state becomes less stable or leakier in EBV-transformed lymphoblastoid cell lines. The cells are also more sensitive to viral reactivation induced by irradiation, which results in the increased production and release of infectious virus, as well as increased susceptibility to ganciclovir treatment. We have identified a target of LMP1-mediated sumoylation that contributes to the maintenance of latency in this context: KRAB-associated protein-1 (KAP1). LMP1 CTAR3-mediated sumoylation regulates the function of KAP1. KAP1 also binds to EBV OriLyt and immediate early promoters in a CTAR3-dependent manner, and inhibition of sumoylation processes abrogates the binding of KAP1 to these promoters. These data provide an additional line of evidence that supports our findings that CTAR3 is a distinct functioning regulatory region of LMP1 and confirm that LMP1-induced sumoylation may help stabilize the maintenance of EBV latency.
Importance: Epstein-Barr virus (EBV) latent membrane protein-1 (LMP1) plays an important role in the maintenance of viral latency. Previously, we documented that LMP1 targets cellular proteins to be modified by a ubiquitin-like protein (SUMO). We have now identified a function for this LMP1-induced modification of cellular proteins in the maintenance of EBV latency. Because latently infected cells have to undergo viral reactivation in order to be vulnerable to antiviral drugs, these findings identify a new way to increase the rate of EBV reactivation, which increases cell susceptibility to antiviral therapies.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
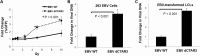
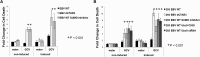


References
-
- Morgan DG, Niederman JC, Miller G, Smith HW, Dowaliby JM. 1979. Site of Epstein-Barr virus replication in the oropharynx. Lancet ii:1154–1157. - PubMed
-
- Pagano J. 2009. EBV diseases, p 217–240. Springer, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

