PI3K/AKT/mTOR pathway plays a major pathogenetic role in glycogen accumulation and tumor development in renal distal tubules of rats and men
- PMID: 25948777
- PMCID: PMC4536997
- DOI: 10.18632/oncotarget.3675
PI3K/AKT/mTOR pathway plays a major pathogenetic role in glycogen accumulation and tumor development in renal distal tubules of rats and men
Abstract
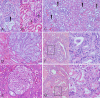
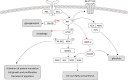
Activation of the PI3K/AKT/mTOR pathway is a crucial molecular event in human clear cell renal cell carcinoma (ccRCC), and is also upregulated in diabetic nephropathy. In diabetic rats metabolic changes affect the renal distal tubular epithelium and lead to glycogen-storing Armanni-Ebstein lesions (AEL), precursor lesions of RCC in the diabetes induced nephrocarcinogenesis model. These lesions resemble human sporadic clear cell tubules (CCT) and tumor cells of human ccRCC.Human sporadic CCT were examined in a collection of 324 nephrectomy specimen, in terms of morphologic, metabolic and molecular alterations, and compared to preneoplastic CCT and RCC developed in the rat following streptozotocin-induced diabetes or N-Nitrosomorpholine administration. Diabetic and non-diabetic rats were subjected to the dual PI3K/mTOR inhibitor, NVP/BEZ235.Human sporadic CCT could be detected in 17.3% of kidney specimens. Human and rat renal CCT display a strong induction of the PI3K/AKT/mTOR pathway and related metabolic alterations. Proteins involved in glycolysis and de novo lipogenesis were upregulated. In in vivo experiments, dual inhibition of PI3K and mTOR resulted in a reduction of proliferation of rat diabetes related CCT and increased autophagic activity.The present data indicate that human sporadic CCT exhibit a pattern of morphologic and metabolic alterations similar to preneoplastic lesions in the rat model. Activation of the PI3K/AKT/mTOR pathway in glycogenotic tubuli is a remarkable molecular event and suggests a preneoplastic character of these lesions also in humans.
Keywords: AKT/mTOR; clear cell tubules; glycogen; nephrocarcinogenesis.
Conflict of interest statement
The authors state that there is no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

