Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection
- PMID: 25948816
- PMCID: PMC4456635
- DOI: 10.4049/jimmunol.1500124
Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection
Abstract
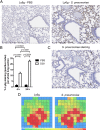
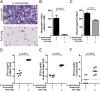
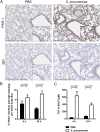
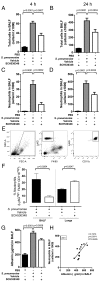
Neutrophils are key effector cells of the innate immune response to pathogenic bacteria, but excessive neutrophilic inflammation can be associated with bystander tissue damage. The mechanisms responsible for neutrophil recruitment to the lungs during bacterial pneumonia are poorly defined. In this study, we focus on the potential role of the major high-affinity thrombin receptor, proteinase-activated receptor 1 (PAR-1), during the development of pneumonia to the common lung pathogen Streptococcus pneumoniae. Our studies demonstrate that neutrophils were indispensable for controlling S. pneumoniae outgrowth but contributed to alveolar barrier disruption. We further report that intra-alveolar coagulation (bronchoalveolar lavage fluid thrombin-antithrombin complex levels) and PAR-1 immunostaining were increased in this model of bacterial lung infection. Functional studies using the most clinically advanced PAR-1 antagonist, SCH530348, revealed a key contribution for PAR-1 signaling in influencing neutrophil recruitment to lung airspaces in response to both an invasive and noninvasive strain of S. pneumoniae (D39 and EF3030) but that PAR-1 antagonism did not impair the ability of the host to control bacterial outgrowth. PAR-1 antagonist treatment significantly decreased pulmonary levels of IL-1β, CXCL1, CCL2, and CCL7 and attenuated alveolar leak. Ab neutralization studies further demonstrated a nonredundant role for IL-1β, CXCL1, and CCL7 in mediating neutrophil recruitment in response to S. pneumoniae infection. Taken together, these data demonstrate a key role for PAR-1 during S. pneumoniae lung infection that is mediated, at least in part, by influencing multiple downstream inflammatory mediators.
Copyright © 2015 The Authors.
Figures


References
-
- Mizgerd J. P. 2006. Lung infection—a public health priority. PLoS Med. 3: e76 Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.00.... - PMC - PubMed
-
- Armstrong G. L., Conn L. A., Pinner R. W. 1999. Trends in infectious disease mortality in the United States during the 20th century. JAMA 281: 61–66. - PubMed
-
- World Health Organisation 2007. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly. Epidemiol. Rec. 82: 93–104. - PubMed
-
- Calbo E., Alsina M., Rodríguez-Carballeira M., Lite J., Garau J. 2010. The impact of time on the systemic inflammatory response in pneumococcal pneumonia. Eur. Respir. J. 35: 614–618. - PubMed
-
- Choi G., Hofstra J.-J. H., Roelofs J. J. T. H., Rijneveld A. W., Bresser P., van der Zee J. S., Florquin S., van der Poll T., Levi M., Schultz M. J. 2008. Antithrombin inhibits bronchoalveolar activation of coagulation and limits lung injury during Streptococcus pneumoniae pneumonia in rats. Crit. Care Med. 36: 204–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

