Effect of manganese exposure on the reproductive organs in immature female rats
- PMID: 25949103
- PMCID: PMC4282234
- DOI: 10.12717/DR.2012.16.4.295
Effect of manganese exposure on the reproductive organs in immature female rats
Abstract
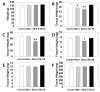
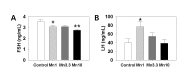
Manganese (Mn(2+)) is a trace element that is essential for normal physiology, and is predominantly obtained from food. Several lines of evidence, however, demonstrated that overexposure to MnCl2 exerts serious neurotoxicity, immunotoxicity and developmental toxicity, particularly in male. The present study aimed to evaluate the effect of 0, 1.0, 3.3, and 10 mg/kg/day doses of MnCl2 on the reproductive organs in the immature female rats. Rats (PND 22; S.D. strain) were exposed to MnCl2 (MnCl2 ∙ 4H2O) dissolved in drinking water for 2 weeks. The animals were sacrificed on PND 35, then the tissues were immediately removed and weighed. Histological studies were performed using the uteri tissue samples. Serum LH and FSH levels were measured with the specific ELISA kits. Body weights of the experimental group animals were not significantly different from those of control group animals. However, ovarian tissue weights in 1 mg and 3.3 mg MnCl2 dose groups were significantly lower than those of control animals (p<0.05 and p<0.01, respectively). Uterine tissue weights of 3.3 mg dose MnCl2 groups were significantly lower than those of control animals (p<0.01), while the 1 mg MnCl2 dose and 10 mg MnCl2 dose failed to induce any change in uterine weight. Similarly, only 3.3 mg MnCl2 dose could induce the significant decrease in the oviduct weight compared to the control group (p<0.05). Non-reproductive tissues such as adrenal and kidney failed to respond to all doses of MnCl2 exposure. The uterine histology revealed that the MnCl2 exposure could affect the myometrial cell proliferation particularly in 3.3 mg dose and 10mg dose group. Serum FSH levels were significantly decreased in 1mg MnCl2 dose and 10 MnCl2 mg groups (p<0.05 and p<0.01, respectively). In contrast, treatment with 1 mg MnCl2 dose induced a significant increment of serum LH level (p<0.05). The present study demonstrated that MnCl2 exposure is capable of inducing abnormal development of reproductive tissues, at least to some extent, and altered gonadotropin secretions in immature female rats. Combined with the well-defined actions of this metal on GnRH and prolactin secretion, one can suggest the Mn(2+) might be a potential environmental mediator which is involved in the female pubertal process.
Keywords: Gonadotropins; Immature female rats; Manganese (Mn2+); Ovary; Pubertal process; Uterus.
Figures

References
-
- Cheng J, Fu J-L, Zhou Z-C. The inhibitory effects of manganese on steroidogenesis in rat primary Leydig cells by disrupting steroidogenic acute regulatory (StAR) protein expression. Toxicology. 2003;187:139–148. - PubMed
-
- Gray LE, Jr, Laskey JW. Multivariate analysis of the effects of manganese on the reproductive physiology and behavior of the male house mouse. J Toxicol Environ Health. 1980;6:861–867. - PubMed
-
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27:765–776. - PubMed
LinkOut - more resources
Full Text Sources

