Optimal Molecular Methods in Detecting p190 (BCR-ABL) Fusion Variants in Hematologic Malignancies: A Case Report and Review of the Literature
- PMID: 25949834
- PMCID: PMC4407518
- DOI: 10.1155/2015/458052
Optimal Molecular Methods in Detecting p190 (BCR-ABL) Fusion Variants in Hematologic Malignancies: A Case Report and Review of the Literature
Abstract
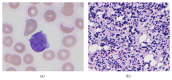
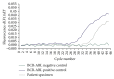
Patients with BCR-ABL1 positive hematologic malignancies and Philadelphia-like B-lymphoblastic leukemia (B-ALL) are potential candidates for targeted therapy with tyrosine kinase inhibitors (TKI). Before TKIs, patients with B-ALL had a much worse prognosis and current treatments with targeted TKI therapy have improved outcomes. Thus, the detection of BCR-ABL1 is crucial and a false negative BCR-ABL1 result may adversely affect patient care. We report a case of a 76-year-old male with a new diagnosis of B-ALL who was initially found to be BCR-ABL1 negative by quantitative polymerase chain reaction (PCR). A concurrent qualitative PCR was performed which detected a positive BCR-ABL1 result that was confirmed by a next generation sequencing (NGS) based assay and identified as the rare fusion variant e1a3 of p190(BCR-ABL). Based on this result, the patient was placed on dasatinib as a targeted therapy. In the era of molecular diagnostic medicine and targeted therapy, it is essential to have an understanding of the limitations of molecular assays and to follow a comprehensive diagnostic approach in order to detect common abnormalities and rare variants. Incorporating NGS methods in an algorithmic manner into the standard diagnostic PCR-based approach for BCR-ABL1 will aid in minimizing false negative results.
Figures

References
-
- Swerdlow S. H., Campo E., Harris N. L., et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th. Lyon, France: International Agency for Research on Cancer (IARC); 2008.
-
- He J., Lipson D., Nahas M., et al. Development and analytical validation of a clinical next generation sequencing-based assay for hematolymphoid malignancies. Proceedings of the American Association for Cancer Research Annual Meeting; April 2014; San Diego, Calif, USA.
-
- LightCycler t(9;22) Quantification Kit Instruction Manual, Version 5. Roche; 2013.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

