Activatable Dendritic 19F Probes for Enzyme Detection
- PMID: 25949857
- PMCID: PMC4416465
- DOI: 10.1021/acsmacrolett.5b00199
Activatable Dendritic 19F Probes for Enzyme Detection
Abstract
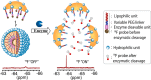
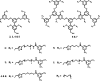
We describe a novel activatable probe for fluorine-19 NMR based on self-assembling amphiphilic dendrons. The dendron probe has been designed to be spectroscopically silent due to the formation of large aggregates. Upon exposure to the specific target enzyme, the aggregates disassemble to give rise to a sharp 19F NMR signal. The probe is capable of detecting enzyme concentrations in the low nanomolar range. Response time of the probe was found to be affected by the hydrophilic-lipophilic balance of dendrons. Understanding the structural factors that underlie this design principle provides the pathway for using this strategy for a broad range of enzyme-based imaging.
Figures


Similar articles
-
Specific detection and imaging of enzyme activity by signal-amplifiable self-assembling (19)F MRI probes.Chemistry. 2013 Sep 16;19(38):12875-83. doi: 10.1002/chem.201300817. Epub 2013 Aug 16. Chemistry. 2013. PMID: 23955524
-
Systematic study of protein detection mechanism of self-assembling 19F NMR/MRI nanoprobes toward rational design and improved sensitivity.J Am Chem Soc. 2011 Aug 3;133(30):11725-31. doi: 10.1021/ja203996c. Epub 2011 Jul 11. J Am Chem Soc. 2011. PMID: 21699190
-
Designing activatable aptamer probes for simultaneous detection of multiple tumor-related proteins in living cancer cells.Biosens Bioelectron. 2015 Jun 15;68:763-770. doi: 10.1016/j.bios.2015.02.004. Epub 2015 Feb 7. Biosens Bioelectron. 2015. PMID: 25682505
-
Nanomaterial-based activatable imaging probes: from design to biological applications.Chem Soc Rev. 2015 Nov 7;44(21):7855-80. doi: 10.1039/c4cs00476k. Chem Soc Rev. 2015. PMID: 26214317 Review.
-
[Development of a Fluorescence Probe for Live Cell Imaging].Yakugaku Zasshi. 2017;137(11):1323-1337. doi: 10.1248/yakushi.17-00132. Yakugaku Zasshi. 2017. PMID: 29093368 Review. Japanese.
Cited by
-
Multichannel dual protein sensing using amphiphilic supramolecular assemblies.Chem Commun (Camb). 2021 Nov 30;57(95):12828-12831. doi: 10.1039/d1cc05407d. Chem Commun (Camb). 2021. PMID: 34787137 Free PMC article.
-
Supramolecular Self-Associations of Amphiphilic Dendrons and Their Properties.Chemistry. 2021 Dec 23;27(72):17976-17998. doi: 10.1002/chem.202102589. Epub 2021 Oct 29. Chemistry. 2021. PMID: 34713506 Free PMC article. Review.
-
Tuning the Reactivity of Micellar Nanoreactors by Precise Adjustments of the Amphiphile and Substrate Hydrophobicity.Macromolecules. 2021 Dec 28;54(24):11419-11426. doi: 10.1021/acs.macromol.1c01755. Epub 2021 Dec 1. Macromolecules. 2021. PMID: 34987270 Free PMC article.
-
19F MRI of Polymer Nanogels Aided by Improved Segmental Mobility of Embedded Fluorine Moieties.Biomacromolecules. 2019 Feb 11;20(2):790-800. doi: 10.1021/acs.biomac.8b01383. Epub 2019 Jan 10. Biomacromolecules. 2019. PMID: 30563327 Free PMC article.
-
Protein-Engineered Nanoscale Micelles for Dynamic 19F Magnetic Resonance and Therapeutic Drug Delivery.ACS Nano. 2019 Mar 26;13(3):2969-2985. doi: 10.1021/acsnano.8b07481. Epub 2019 Feb 19. ACS Nano. 2019. PMID: 30758189 Free PMC article.
References
-
- Weissleder R.; Tung C. H.; Mahmood U.; Bogdanov A. Nat. Biotechnol. 1999, 17, 375. - PubMed
- Chen J.; Tung C.-H.; Allport J. R.; Chen S.; Weissleder R.; Huang P. L. Circulation 2005, 111, 1800. - PMC - PubMed
- Hu H.-Y.; Gehrig S.; Reither G.; Subramanian D.; Mall M. A.; Plettenburg O.; Schultz C. Biotechnol. J. 2014, 9, 266. - PubMed
- Gao W.; Xing B.; Tsien R. Y.; Rao J. J. Am. Chem. Soc. 2003, 125, 11146. - PubMed
- Kamiya M.; Kobayashi H.; Hama Y.; Koyama Y.; Bernardo M.; Nagano T.; Choyke P. L.; Urano Y. J. Am. Chem. Soc. 2007, 129, 3918. - PMC - PubMed
- Silvers W. C.; Prasai B.; Burk D. H.; Brown M. L.; McCarley R. L. J. Am. Chem. Soc. 2013, 135, 309. - PMC - PubMed
-
- Perez J. M.; Josephson L.; O’Loughlin T.; Högemann D.; Weissleder R. Nat. Biotechnol. 2002, 20, 816. - PubMed
- Louie A. Y.; Huber M. M.; Ahrens E. T.; Rothbacher U.; Moats R.; Jacobs R. E.; Fraser S. E.; Meade T. J. Nat. Biotechnol. 2000, 18, 321. - PubMed
- Yang C.-T.; Chuang K.-H. Med. Chem. Commun. 2012, 3, 552.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

