MHC-I modulation due to changes in tumor cell metabolism regulates tumor sensitivity to CTL and NK cells
- PMID: 25949869
- PMCID: PMC4368123
- DOI: 10.4161/2162402X.2014.985924
MHC-I modulation due to changes in tumor cell metabolism regulates tumor sensitivity to CTL and NK cells
Abstract
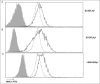
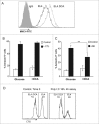
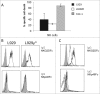
Tumor cells have a tendency to use glucose fermentation to obtain energy instead of mitochondrial oxidative phosphorylation (OXPHOS). We demonstrated that this phenotype correlated with loss of ERK5 expression and with reduced MHC class I expression. Consequently, tumor cells could evade cytotoxic T lymphocyte (CTL)-mediated immune surveillance, but also increase their sensitivity to natural killer (NK) cells. These outcomes were evaluated using two cellular models: leukemic EL4 cells and L929 transformed fibroblasts and their derived ρ° cell lines, which lack mitochondrial DNA. We have also used a L929 cell sub-line that spontaneously lost matrix attachment (L929dt), reminiscent of metastasis generation, that also downregulated MHC-I and ERK5 expression. MHC-I expression is lower in ρ° cells than in the parental cell lines, but they were equally sensitive to CTL. On the contrary, ρ° cells were more sensitive to activated NK cells than parental cells. On the other hand, L929dt cells were resistant to CTL and NK cells, showed reduced viability when forced to perform OXPHOS, and surviving cells increased MHC-I expression and became sensitive to CTL. The present results suggest that when the reduction in MHC-I levels in tumor cells due to glycolytic metabolism is partial, the increase in sensitivity to NK cells seems to predominate. However, when tumor cells completely lose MHC-I expression, the combination of treatments that increase OXPHOS with CTL-mediated immunotherapy could be a promising therapeutic approach.
Keywords: CTG; NK cells; cancer immunotherapy; cell tracker green; CTL; cytotoxic T lymphocyte; DCA; cytotoxic T lymphocytes; deoxy-ribonucleic acid; ERK5; dichloroacetate; dicholoroacetate; DNA; extracellular regulated kinase 5; FCS; fetal calf serum; mAb; glucose metabolism; magnetic cell separation; MHC-I; major histocompatibility complex class I; mRNA; messenger ribonucleic acid; NK; monoclonal antibody; MACS; natural killer; OXPHOS; oxidative phoshorylation; PBS; phosphate buffered saline; Poly I:C; polyinosinic: cytidilic acid; RNA; ribonucleic acid; sh; small hairpin; ρ° cells.
Figures






References
-
- Warburg O. On respiratory impairment in cancer cells. Science 1956; 124:269y-70; PMID: - PubMed
-
- Charni S, de Bettignies G, Rathore MG, Aguiló JI, van den Elsen PJ, Haouzi D, Hipskind RA, Enriquez JA, Sanchez-Beato M, Pardo J, et al. Oxidative phosphorylation induces de novo expression of the MHC class I in tumor cells through the ERK5 pathway. J Immunol 2010; 185:3498-503; PMID:; http://dx.doi.org/10.4049/jimmunol.1001250 - DOI - PubMed
-
- Dunn C, Wiltshire C, MacLaren A, Gillespie DAF. Molecular mechanism and biological function of JNK signaling via the c-Jun transcription factor. Cell Signal 2002; 14:585-93; PMID:; http://dx.doi.org/10.1016/S0898-6568(01)00275-3 - DOI - PubMed
-
- Villalba M, Rathore MG, López-Royuela N, Krzywinska E, Garaude J, Allende-Vega N. From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol 2013; 45:106-13; PMID:; http://dx.doi.org/10.1016/j.biocel.2012.04.024 - DOI - PubMed
-
- Garaude J, Kaminski S, Charni S, Aguiló JI, Jacquet C, Plays M, Rodriguez F, Hernández J, Hipskind RA, Anel A, et al. Impaired anti-leukemic immune response in PKCq-deficient mice. Mol Immunol 2008; 45:3463-9; PMID:; http://dx.doi.org/10.1016/j.molimm.2008.03.016 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
