Using "Tender" X-ray Ambient Pressure X-Ray Photoelectron Spectroscopy as A Direct Probe of Solid-Liquid Interface
- PMID: 25950241
- PMCID: PMC4650780
- DOI: 10.1038/srep09788
Using "Tender" X-ray Ambient Pressure X-Ray Photoelectron Spectroscopy as A Direct Probe of Solid-Liquid Interface
Abstract
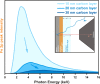
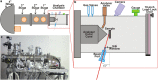
We report a new method to probe the solid-liquid interface through the use of a thin liquid layer on a solid surface. An ambient pressure XPS (AP-XPS) endstation that is capable of detecting high kinetic energy photoelectrons (7 keV) at a pressure up to 110 Torr has been constructed and commissioned. Additionally, we have deployed a "dip &pull" method to create a stable nanometers-thick aqueous electrolyte on platinum working electrode surface. Combining the newly constructed AP-XPS system, "dip &pull" approach, with a "tender" X-ray synchrotron source (2 keV-7 keV), we are able to access the interface between liquid and solid dense phases with photoelectrons and directly probe important phenomena occurring at the narrow solid-liquid interface region in an electrochemical system. Using this approach, we have performed electrochemical oxidation of the Pt electrode at an oxygen evolution reaction (OER) potential. Under this potential, we observe the formation of both Pt(2+) and Pt(4+) interfacial species on the Pt working electrode in situ. We believe this thin-film approach and the use of "tender" AP-XPS highlighted in this study is an innovative new approach to probe this key solid-liquid interface region of electrochemistry.
Figures




References
-
- Hűfner S. Photoelectron Spectroscopy Principles and Applications. Springer 2003).
-
- Siegbahn K. ESCA applied to free molecules. North-Holland Pub. Co. 1969).
-
- Siegbahn H. Electron Spectroscopy for Chemical Analysis of Liquids and Solutions. J. Phys. Chem. B 89, 897 (1985).
-
- Frank Ogletree D., Bluhm H., Hebenstreit E. D. & Salmeron M. Photoelectron spectroscopy under ambient pressure and temperature conditions. Nucl. Instrum. Meth. A. 601, 151–160, doi: 10.1016/j.nima.2008.12.155 (2009). - DOI
-
- Salmeron M. & Schlögl R. Ambient pressure photoelectron spectroscopy: A new tool for surface science and nanotechnology. Surf. Sci. Rep. 63, 169–199 (2008).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

