IL-1 signaling inhibits Trichophyton rubrum conidia development and modulates the IL-17 response in vivo
- PMID: 25950847
- PMCID: PMC4601166
- DOI: 10.1080/21505594.2015.1020274
IL-1 signaling inhibits Trichophyton rubrum conidia development and modulates the IL-17 response in vivo
Abstract
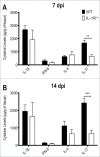
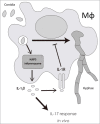
Dermatophytosis are one of the most common fungal infections in the world. They compromise keratinized tissues and the main etiological agent is Trichophyton rubrum. Macrophages are key cells in innate immunity and prominent sources of IL-1β, a potent inflammatory cytokine whose main production pathway is by the activation of inflammasomes and caspase-1. However, the role of inflammasomes and IL-1 signaling against T.rubrum has not been reported. In this work, we observed that bone marrow-derived macrophages produce IL-1β in response to T.rubrum conidia in a NLRP3-, ASC- and caspase-1-dependent fashion. Curiously, lack of IL-1 signaling promoted hyphae development, uncovering a protective role for IL-1β in macrophages. In addition, mice lacking IL-1R showed reduced IL-17 production, a key cytokine in the antifungal defense, in response to T.rubrum. Our findings point to a prominent role of IL-1 signaling in the immune response to T.rubrum, opening the venue for the study of this pathway in other fungal infections.
Keywords: ASC, Apoptosis-associated Speck-like protein containing a CARD; BMDM, bone marrow derived macrophages; CFU, colony-forming units; IL, interleukin; IL-1 signaling; IL-17; NLR; NLRP3 inflammasom; NLRP3, NOD-like receptor family, pyrin domain containing 3; NOD-like receptor; Trichophyton rubrum; WT; dermatophytosis; fungal infections; innate immunity; wild-type.
Figures



Similar articles
-
IFN-γ impairs Trichophyton rubrum proliferation in a murine model of dermatophytosis through the production of IL-1β and reactive oxygen species.Med Mycol. 2014 Apr;52(3):293-302. doi: 10.1093/mmy/myt011. Epub 2014 Feb 13. Med Mycol. 2014. PMID: 24577006
-
Dectin-1 and Dectin-2 promote control of the fungal pathogen Trichophyton rubrum independently of IL-17 and adaptive immunity in experimental deep dermatophytosis.Innate Immun. 2016 Jul;22(5):316-24. doi: 10.1177/1753425916645392. Epub 2016 Apr 26. Innate Immun. 2016. PMID: 27189427
-
Monocyte-derived dendritic cells from patients with dermatophytosis restrict the growth of Trichophyton rubrum and induce CD4-T cell activation.PLoS One. 2014 Nov 5;9(11):e110879. doi: 10.1371/journal.pone.0110879. eCollection 2014. PLoS One. 2014. PMID: 25372145 Free PMC article.
-
The Immunologic Response to Trichophyton Rubrum in Lower Extremity Fungal Infections.J Fungi (Basel). 2015 Jul 17;1(2):130-137. doi: 10.3390/jof1020130. J Fungi (Basel). 2015. PMID: 29376904 Free PMC article. Review.
-
[Innate receptors and IL-17 in the immune response against human pathogenic fungi].Rev Fac Cien Med Univ Nac Cordoba. 2016;73(3):188-196. Rev Fac Cien Med Univ Nac Cordoba. 2016. PMID: 27805556 Review. Spanish.
Cited by
-
Human primary epidermal organoids enable modeling of dermatophyte infections.Cell Death Dis. 2021 Jan 4;12(1):35. doi: 10.1038/s41419-020-03330-y. Cell Death Dis. 2021. PMID: 33414472 Free PMC article.
-
Extracellular Vesicles From the Dermatophyte Trichophyton interdigitale Modulate Macrophage and Keratinocyte Functions.Front Immunol. 2018 Oct 9;9:2343. doi: 10.3389/fimmu.2018.02343. eCollection 2018. Front Immunol. 2018. PMID: 30356863 Free PMC article.
-
Relevant Animal Models in Dermatophyte Research.Mycopathologia. 2017 Feb;182(1-2):229-240. doi: 10.1007/s11046-016-0079-3. Epub 2016 Oct 11. Mycopathologia. 2017. PMID: 27730454 Review.
-
Dermatophyte infection: from fungal pathogenicity to host immune responses.Front Immunol. 2023 Nov 2;14:1285887. doi: 10.3389/fimmu.2023.1285887. eCollection 2023. Front Immunol. 2023. PMID: 38022599 Free PMC article. Review.
-
Dermatophytosis in context of Trichophyton rubrum: host defense mechanisms, virulence factors, and treatment innovations.Mol Biol Rep. 2025 Jun 24;52(1):634. doi: 10.1007/s11033-025-10744-4. Mol Biol Rep. 2025. PMID: 40553313 Review.
References
-
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol 2010; 10:117; http://dx.doi.org/10.1038/nri2691 - DOI - PubMed
-
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012; 481:278-86; PMID:22258606; http://dx.doi.org/10.1038/nature10759 - DOI - PubMed
-
- Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012; 13:325-32; PMID:22430785; http://dx.doi.org/10.1038/ni.2231 - DOI - PMC - PubMed
-
- Horvath GL, Schrum JE, De Nardo CM, Latz E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol Rev 2011; 243:119-35; PMID:21884172; http://dx.doi.org/10.1111/j.1600-065X.2011.01050.x - DOI - PMC - PubMed
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009; 27:519-50; PMID:19302047; http://dx.doi.org/10.1146/annurev.immunol.021908.132612 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
