Catenanes: fifty years of molecular links
- PMID: 25951013
- PMCID: PMC4515087
- DOI: 10.1002/anie.201411619
Catenanes: fifty years of molecular links
Abstract
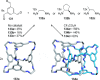
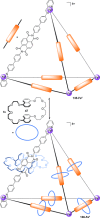
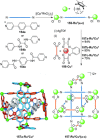
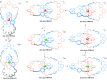
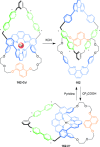
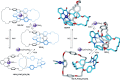
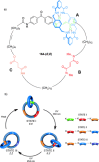
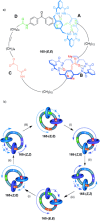
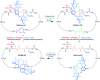
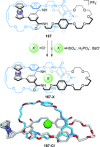
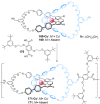
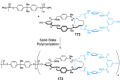
Half a century after Schill and Lüttringhaus carried out the first directed synthesis of a [2]catenane, a plethora of strategies now exist for the construction of molecular Hopf links (singly interlocked rings), the simplest type of catenane. The precision and effectiveness with which suitable templates and/or noncovalent interactions can arrange building blocks has also enabled the synthesis of intricate and often beautiful higher order interlocked systems, including Solomon links, Borromean rings, and a Star of David catenane. This Review outlines the diverse strategies that exist for synthesizing catenanes in the 21st century and examines their emerging applications and the challenges that still exist for the synthesis of more complex topologies.
Keywords: catenanes; interlocked molecules; links; supramolecular chemistry; template synthesis.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
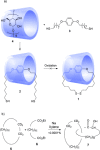
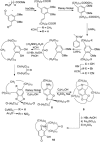
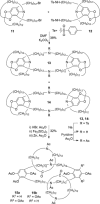
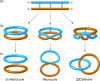
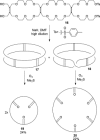

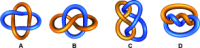
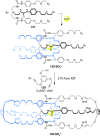
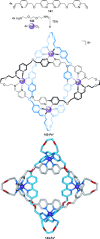
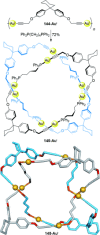
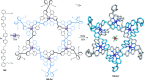
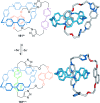
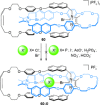
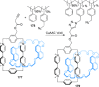
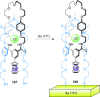
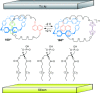
 ).
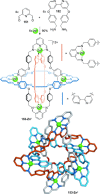
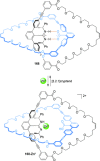
).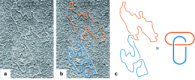
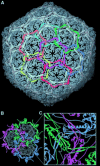

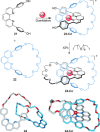
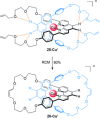
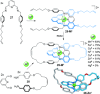
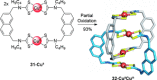
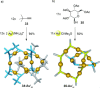
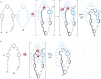
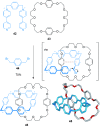
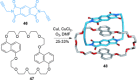
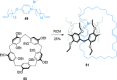
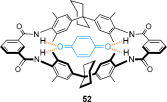
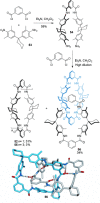
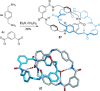
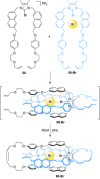
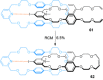
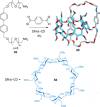
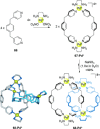
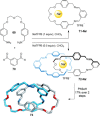
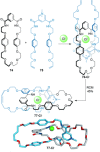
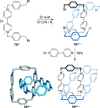
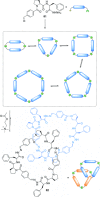
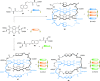
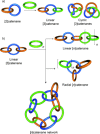
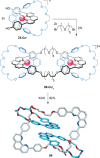
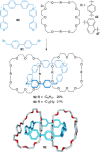
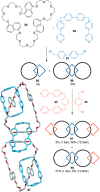
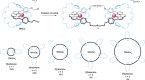
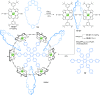
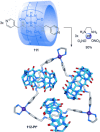
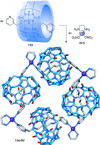
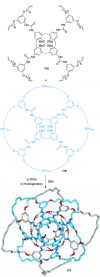
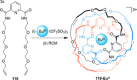
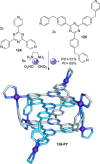
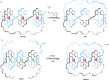
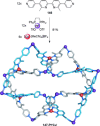
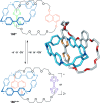
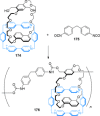
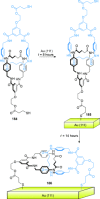
 link.[
link.[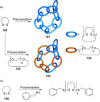

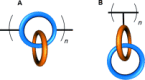


References
-
- Wasserman E. J. Am. Chem. Soc. 1960;82:4433–4434.
-
- Wasserman E. Sci. Am. 1962;207:94–102.
-
- Frisch HL, Wasserman E. J. Am. Chem. Soc. 1961;83:3789–3795.
-
- The cyclic nature of many of the first macrocycles to be synthesized went unappreciated for decades. In an early example, Posner condensed 2-aminobenzaldehyde in the presence of ZnII. in 1898.
-
- Posner T. Ber. Dtsch. Chem. Ges. 1898;31:656–660. but the isolated product was not recognized to be a macrocycle for another 30 years.
LinkOut - more resources
Full Text Sources
Other Literature Sources

