Interleukin-15 Dendritic Cells Harness NK Cell Cytotoxic Effector Function in a Contact- and IL-15-Dependent Manner
- PMID: 25951230
- PMCID: PMC4423923
- DOI: 10.1371/journal.pone.0123340
Interleukin-15 Dendritic Cells Harness NK Cell Cytotoxic Effector Function in a Contact- and IL-15-Dependent Manner
Abstract
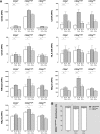
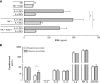
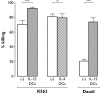
The contribution of natural killer (NK) cells to the treatment efficacy of dendritic cell (DC)-based cancer vaccines is being increasingly recognized. Much current efforts to optimize this form of immunotherapy are therefore geared towards harnessing the NK cell-stimulatory ability of DCs. In this study, we investigated whether generation of human monocyte-derived DCs with interleukin (IL)-15 followed by activation with a Toll-like receptor stimulus endows these DCs, commonly referred to as "IL-15 DCs", with the capacity to stimulate NK cells. In a head-to-head comparison with "IL-4 DCs" used routinely for clinical studies, IL-15 DCs were found to induce a more activated, cytotoxic effector phenotype in NK cells, in particular in the CD56bright NK cell subset. With the exception of GM-CSF, no significant enhancement of cytokine/chemokine secretion was observed following co-culture of NK cells with IL-15 DCs. IL-15 DCs, but not IL-4 DCs, promoted NK cell tumoricidal activity towards both NK-sensitive and NK-resistant targets. This effect was found to require cell-to-cell contact and to be mediated by DC surface-bound IL-15. This study shows that DCs can express a membrane-bound form of IL-15 through which they enhance NK cell cytotoxic function. The observed lack of membrane-bound IL-15 on "gold-standard" IL-4 DCs and their consequent inability to effectively promote NK cell cytotoxicity may have important implications for the future design of DC-based cancer vaccine studies.
Conflict of interest statement
Figures

Similar articles
-
Transpresentation of interleukin-15 by IL-15/IL-15Rα mRNA-engineered human dendritic cells boosts antitumoral natural killer cell activity.Oncotarget. 2015 Dec 29;6(42):44123-33. doi: 10.18632/oncotarget.6536. Oncotarget. 2015. PMID: 26675759 Free PMC article.
-
Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs.Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16606-11. doi: 10.1073/pnas.0407522101. Epub 2004 Nov 9. Proc Natl Acad Sci U S A. 2004. PMID: 15536127 Free PMC article.
-
Helper activity of natural killer cells during the dendritic cell-mediated induction of melanoma-specific cytotoxic T cells.J Immunother. 2011 Apr;34(3):270-8. doi: 10.1097/CJI.0b013e31820b370b. J Immunother. 2011. PMID: 21389871 Free PMC article.
-
Natural killer-dendritic cell cross-talk in cancer immunotherapy.Expert Opin Biol Ther. 2005 Oct;5(10):1303-15. doi: 10.1517/14712598.5.10.1303. Expert Opin Biol Ther. 2005. PMID: 16197336 Review.
-
The bidirectional crosstalk between human dendritic cells and natural killer cells.J Innate Immun. 2011;3(3):258-63. doi: 10.1159/000323923. Epub 2011 Mar 11. J Innate Immun. 2011. PMID: 21411969 Review.
Cited by
-
CCL22 mutations drive natural killer cell lymphoproliferative disease by deregulating microenvironmental crosstalk.Nat Genet. 2022 May;54(5):637-648. doi: 10.1038/s41588-022-01059-2. Epub 2022 May 5. Nat Genet. 2022. PMID: 35513723 Free PMC article.
-
Research progress on dendritic cell vaccines in cancer immunotherapy.Exp Hematol Oncol. 2022 Jan 24;11(1):3. doi: 10.1186/s40164-022-00257-2. Exp Hematol Oncol. 2022. PMID: 35074008 Free PMC article. Review.
-
The Characteristics of Tumor Microenvironment in Triple Negative Breast Cancer.Cancer Manag Res. 2022 Jan 3;14:1-17. doi: 10.2147/CMAR.S316700. eCollection 2022. Cancer Manag Res. 2022. PMID: 35018117 Free PMC article. Review.
-
Complexed hyaluronic acid-based nanoparticles in cancer therapy and diagnosis: Research trends by natural language processing.Heliyon. 2024 Dec 18;11(1):e41246. doi: 10.1016/j.heliyon.2024.e41246. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39811313 Free PMC article.
-
Memory-like NK cell differentiation, inhibitory NKG2A blockade, and improved recognition via antibody or CAR engineering combine to enhance NK cell attack against multiple myeloma.J Immunol. 2025 Jan 1;214(1):1-11. doi: 10.1093/jimmun/vkae004. J Immunol. 2025. PMID: 40073259
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

