Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis
- PMID: 25951291
- PMCID: PMC4440623
- DOI: 10.1021/acs.accounts.5b00068
Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis
Abstract
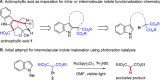
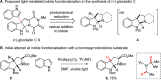
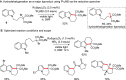
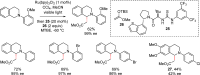
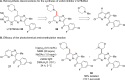
While the use of visible light to drive chemical reactivity is of high importance to the development of environmentally benign chemical transformations, the concomitant use of a stoichiometric electron donor or acceptor is often required to steer the desired redox behavior of these systems. The low-cost and ubiquity of tertiary amine bases has led to their widespread use as reductive additives in photoredox catalysis. Early use of trialkylamines in this context was focused on their role as reductive excited state quenchers of the photocatalyst, which in turn provides a more highly reducing catalytic intermediate. In this Account, we discuss some of the observations and thought processes that have led from our use of amines as reductive additives to their use as complex substrates and intermediates for natural product synthesis. Early attempts by our group to construct key carbon-carbon bonds via free-radical intermediates led to the observation that some trialkylamines readily behave as efficient hydrogen atom donors under redox-active photochemical conditions. In the wake of in-depth mechanistic studies published in the 1970s, 1980s and 1990s, this understanding has in turn allowed for a systematic approach to the design of a number of photochemical methodologies through rational tuning of the amine component. Minimization of the C-H donicity of the amine additive was found to promote desired C-C bond formation in a number of contexts, and subsequent elucidation of the amine's redox fate has sparked a reevaluation of the amine's role from that of reagent to that of substrate. The reactivity of tertiary amines in these photochemical systems is complex, and allows for a number of mechanistic possibilities that are not necessarily mutually exclusive. A variety of combinations of single-electron oxidation, C-H abstraction, deprotonation, and β-scission result in the formation of reactive intermediates such as α-amino radicals and iminium ions. These processes have been explored in depth in the photochemical literature and have resulted in a firm mechanistic grasp of the behavior of amine radical cations in fundamental systems. Harnessing the synthetic potential of these transient species represents an ongoing challenge for the controlled functionalization of amine substrates, because these mechanistic possibilities may result in undesired byproduct formation or substrate decomposition. The presence of tertiary amines in numerous alkaloids, pharmaceuticals, and agrochemicals lends credence to the potential utility of this chemistry in natural product synthesis, and herein we will discuss how these transformations might be controlled for synthetic purposes.
Figures









References
-
- Kärkäs M. D.; Verho O.; Johnston E. V.; Åkermark B. Artificial Photosynthesis: Molecular Systems for Catalytic Water Oxidation. Chem. Rev. 2014, 114, 11863–12001. - PubMed
-
- Morris A. J.; Meyer G. J.; Fujita E. Molecular Approaches to the Photocatalytic Reduction of Carbon Dioxide for Solar Fuels. Acc. Chem. Res. 2009, 42, 1983–1994. - PubMed
-
- Juris A.; Balzani V.; Barigelletti F.; Campagna S.; Belser P.; von Zelewski A. Ru(II) Poylpyridine Complexes: Photophysics, Photochemistry, Electrochemistry, and Chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277.
-
- Ischay M. A.; Anzovino M. E.; Du J.; Yoon T. P. Efficient Visible Light Photocatalysis of [2 + 2] Enone Cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

