Coordination of peptidoglycan synthesis and outer membrane constriction during Escherichia coli cell division
- PMID: 25951518
- PMCID: PMC4458516
- DOI: 10.7554/eLife.07118
Coordination of peptidoglycan synthesis and outer membrane constriction during Escherichia coli cell division
Abstract
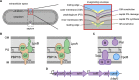
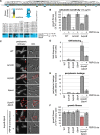
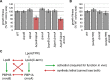
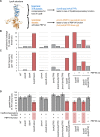
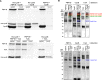
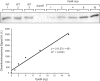
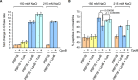
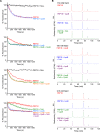
To maintain cellular structure and integrity during division, Gram-negative bacteria must carefully coordinate constriction of a tripartite cell envelope of inner membrane, peptidoglycan (PG), and outer membrane (OM). It has remained enigmatic how this is accomplished. Here, we show that envelope machines facilitating septal PG synthesis (PBP1B-LpoB complex) and OM constriction (Tol system) are physically and functionally coordinated via YbgF, renamed CpoB (Coordinator of PG synthesis and OM constriction, associated with PBP1B). CpoB localizes to the septum concurrent with PBP1B-LpoB and Tol at the onset of constriction, interacts with both complexes, and regulates PBP1B activity in response to Tol energy state. This coordination links PG synthesis with OM invagination and imparts a unique mode of bifunctional PG synthase regulation by selectively modulating PBP1B cross-linking activity. Coordination of the PBP1B and Tol machines by CpoB contributes to effective PBP1B function in vivo and maintenance of cell envelope integrity during division.
Keywords: E. coli; cell division; cell envelope; infectious disease; microbiology; outer membrane; peptidoglycan.
Conflict of interest statement
The other authors declare that no competing interests exist.
Figures










References
-
- Banzhaf M, van den Berg van Saparoea B, Terrak M, Fraipont C, Egan A, Philippe J, Zapun A, Breukink E, Nguyen-Disteche M, den Blaauwen T, Vollmer W. Cooperativity of peptidoglycan synthases active in bacterial cell elongation. Molecular Microbiology. 2012;85:179–194. doi: 10.1111/j.1365-2958.2012.08103.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

