Topological control of the Caulobacter cell cycle circuitry by a polarized single-domain PAS protein
- PMID: 25952018
- PMCID: PMC4432633
- DOI: 10.1038/ncomms8005
Topological control of the Caulobacter cell cycle circuitry by a polarized single-domain PAS protein
Abstract
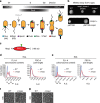
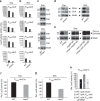
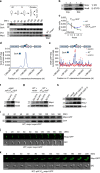
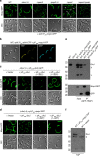
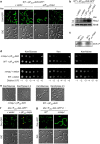
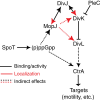
Despite the myriad of different sensory domains encoded in bacteria, only a few types are known to control the cell cycle. Here we use a forward genetic screen for Caulobacter crescentus motility mutants to identify a conserved single-domain PAS (Per-Arnt-Sim) protein (MopJ) with pleiotropic regulatory functions. MopJ promotes re-accumulation of the master cell cycle regulator CtrA after its proteolytic destruction is triggered by the DivJ kinase at the G1-S transition. MopJ and CtrA syntheses are coordinately induced in S-phase, followed by the sequestration of MopJ to cell poles in Caulobacter. Polarization requires Caulobacter DivJ and the PopZ polar organizer. MopJ interacts with DivJ and influences the localization and activity of downstream cell cycle effectors. Because MopJ abundance is upregulated in stationary phase and by the alarmone (p)ppGpp, conserved systemic signals acting on the cell cycle and growth phase control are genetically integrated through this conserved single PAS-domain protein.
Figures
Similar articles
-
Convergence of alarmone and cell cycle signaling from trans-encoded sensory domains.mBio. 2015 Oct 20;6(5):e01415-15. doi: 10.1128/mBio.01415-15. mBio. 2015. PMID: 26489861 Free PMC article.
-
In-phase oscillation of global regulons is orchestrated by a pole-specific organizer.Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12550-12555. doi: 10.1073/pnas.1610723113. Epub 2016 Oct 17. Proc Natl Acad Sci U S A. 2016. PMID: 27791133 Free PMC article.
-
Polar Localization Hub Protein PopZ Restrains Adaptor-Dependent ClpXP Proteolysis in Caulobacter crescentus.J Bacteriol. 2018 Sep 24;200(20):e00221-18. doi: 10.1128/JB.00221-18. Print 2018 Oct 15. J Bacteriol. 2018. PMID: 30082457 Free PMC article.
-
A bacterial cell-cycle regulatory network operating in time and space.Science. 2003 Sep 26;301(5641):1874-7. doi: 10.1126/science.1087694. Science. 2003. PMID: 14512618 Review.
-
DNA methylation in Caulobacter and other Alphaproteobacteria during cell cycle progression.Trends Microbiol. 2014 Sep;22(9):528-35. doi: 10.1016/j.tim.2014.05.003. Epub 2014 Jun 2. Trends Microbiol. 2014. PMID: 24894626 Review.
Cited by
-
Computational modeling of unphosphorylated CtrA:Cori binding in the Caulobacter cell cycle.iScience. 2021 Nov 10;24(12):103413. doi: 10.1016/j.isci.2021.103413. eCollection 2021 Dec 17. iScience. 2021. PMID: 34901785 Free PMC article.
-
PAS domains in bacterial signal transduction.Curr Opin Microbiol. 2021 Jun;61:8-15. doi: 10.1016/j.mib.2021.01.004. Epub 2021 Feb 26. Curr Opin Microbiol. 2021. PMID: 33647528 Free PMC article. Review.
-
Crosstalk Regulation Between Bacterial Chromosome Replication and Chromosome Partitioning.Front Microbiol. 2019 Feb 26;10:279. doi: 10.3389/fmicb.2019.00279. eCollection 2019. Front Microbiol. 2019. PMID: 30863373 Free PMC article. Review.
-
Bacterial cell cycle control by citrate synthase independent of enzymatic activity.Elife. 2020 Mar 9;9:e52272. doi: 10.7554/eLife.52272. Elife. 2020. PMID: 32149608 Free PMC article.
-
An intracellular compass spatially coordinates cell cycle modules in Caulobacter crescentus.Curr Opin Microbiol. 2016 Oct;33:131-139. doi: 10.1016/j.mib.2016.06.007. Epub 2016 Aug 10. Curr Opin Microbiol. 2016. PMID: 27517351 Free PMC article. Review.
References
-
- Skerker J. M. & Laub M. T. Cell-cycle progression and the generation of asymmetry in Caulobacter crescentus. Nat. Rev. Microbiol. 2, 325–337 (2004). - PubMed
-
- Quon K. C., Marczynski G. T. & Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell 84, 83–93 (1996). - PubMed
-
- Ely B. Genetics of Caulobacter crescentus. Methods Enzymol. 204, 372–384 (1991). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

