Multispectral imaging reveals the tissue distribution of tetraspanins in human lymphoid organs
- PMID: 25952155
- PMCID: PMC4522275
- DOI: 10.1007/s00418-015-1326-2
Multispectral imaging reveals the tissue distribution of tetraspanins in human lymphoid organs
Abstract
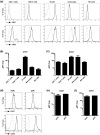
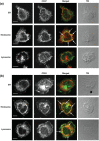
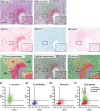
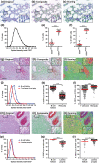
Multispectral imaging is a novel microscopy technique that combines imaging with spectroscopy to obtain both quantitative expression data and tissue distribution of different cellular markers. Tetraspanins CD37 and CD53 are four-transmembrane proteins involved in cellular and humoral immune responses. However, comprehensive immunohistochemical analyses of CD37 and CD53 in human lymphoid organs have not been performed so far. We investigated CD37 and CD53 protein expression on primary human immune cell subsets in blood and in primary and secondary lymphoid organs. Both tetraspanins were prominently expressed on antigen-presenting cells, with highest expression of CD37 on B lymphocytes. Analysis of subcellular distribution showed presence of both tetraspanins on the plasma membrane and on endosomes. In addition, CD53 was also present on lysosomes. Quantitative analysis of expression and localization of CD37 and CD53 on lymphocytes within lymphoid tissues by multispectral imaging revealed high expression of both tetraspanins on CD20(+) cells in B cell follicles in human spleen and appendix. CD3(+) T cells within splenic T cell zones expressed lower levels of CD37 and CD53 compared to T cells in the red pulp of human spleen. B cells in human bone marrow highly expressed CD37, whereas the expression of CD53 was low. In conclusion, we demonstrate differential expression of CD37 and CD53 on primary human immune cells, their subcellular localization and their quantitative distribution in human lymphoid organs. This study provides a solid basis for better insight into the function of tetraspanins in the human immune response.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

