The BCL-2 protein family, BH3-mimetics and cancer therapy
- PMID: 25952548
- PMCID: PMC4572872
- DOI: 10.1038/cdd.2015.50
The BCL-2 protein family, BH3-mimetics and cancer therapy
Abstract
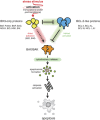
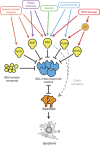
Escape from apoptosis is a key attribute of tumour cells and facilitates chemo-resistance. The 'BCL-2-regulated' or 'intrinsic' apoptotic pathway integrates stress and survival signalling to govern whether a cancer cell will live or die. Indeed, many pro-apoptotic members of the BCL-2 family have demonstrated tumour-suppression activity in mouse models of cancer and are lost or repressed in certain human cancers. Conversely, overexpression of pro-survival BCL-2 family members promotes tumorigenesis in humans and in mouse models. Many of the drugs currently used in the clinic mediate their therapeutic effects (at least in part) through the activation of the BCL-2-regulated apoptotic pathway. However, initiators of this apoptotic pathway, such as p53, are mutated, lost or silenced in many human cancers rendering them refractory to treatment. To counter such resistance mechanisms, a novel class of therapeutics, 'BH3-mimetics', has been developed. These drugs directly activate apoptosis by binding and inhibiting select antiapoptotic BCL-2 family members and thereby bypass the requirement for upstream initiators, such as p53. In this review, we discuss the role of the BCL-2 protein family in the development and treatment of cancer, with an emphasis on mechanistic studies using well-established mouse models of cancer, before describing the development and already recognised potential of the BH3-mimetic compounds.
Figures
References
-
- 1Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Ann Rev Biochem 2000; 69: 217–245. - PubMed
-
- 3Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. - PubMed
-
- 5Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014; 157: 1013–1022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

