Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/ hydroxyapatite/collagen scaffolds
- PMID: 25952675
- PMCID: PMC4429830
- DOI: 10.1186/s12967-015-0499-8
Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/ hydroxyapatite/collagen scaffolds
Abstract
Background: Osteoinduction and proliferation of bone-marrow stromal cells (BMSCs) in three-dimensional (3D) poly(ε-caprolactone) (PCL) scaffolds have not been studied throughly and are technically challenging. This study aimed to optimize nanocomposites of 3D PCL scaffolds to provide superior adhesion, proliferation and differentiation environment for BMSCs in this scenario.
Methods: BMSCs were isolated and cultured in a novel 3D tissue culture poly(ε-caprolactone) (PCL) scaffold coated with poly-lysine, hydroxyapatite (HAp), collagen and HAp/collagen. Cell morphology was observed and BMSC biomarkers for osteogenesis, osteoblast differentiation and activation were analyzed.
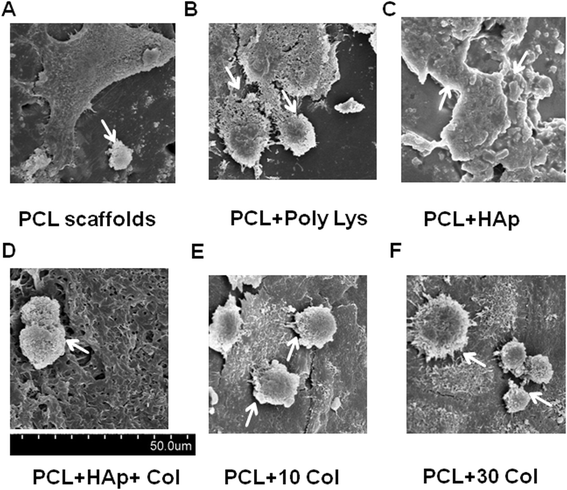
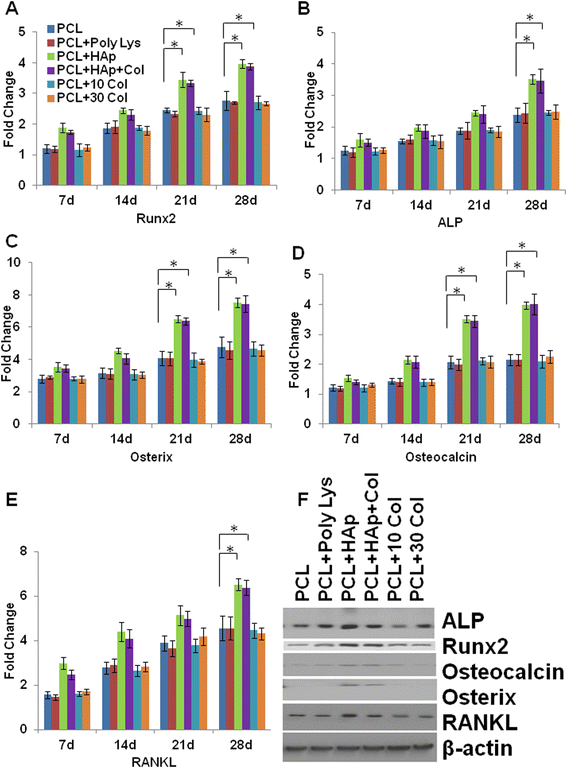
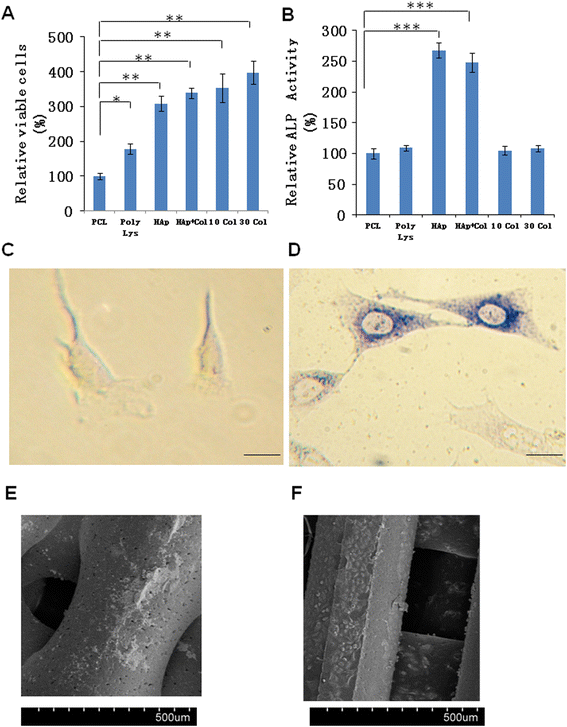
Results: Scanning Electron Microscope (SEM) micrographs showed that coating materials were uniformly deposited on the surface of PCL scaffolds and BMSCs grew and aggregated to form clusters during 3D culture. Both mRNA and protein levels of the key players of osteogenesis and osteoblast differentiation and activation, including runt-related transcription factor 2 (Runx2), alkaline phosphates (ALP), osterix, osteocalcin, and RANKL, were significantly higher in BMSCs seeded in PCL scaffolds coated with HAp or HAp/collagen than those seeded in uncoated PCL scaffolds, whereas the expression levels were not significantly different in collagen or poly-lysine coated PCL scaffolds. In addition, poly-lysine, collagen, HAp/collagen, and HAp coated PCL scaffolds had significantly more viable cells than uncoated PCL scaffolds, especially scaffolds with HAp/collagen and collagen-alone coatings. That BMSCs in HAp or HAp/collagen PCL scaffolds had remarkably higher ALP activities than those in collagen-coated alone or uncoated PCL scaffolds indicating higher osteogenic differentiation levels of BMSCs in HAp or HAp/collagen PCL scaffolds. Moreover, morphological changes of BMSCs after four-week of 3D culture confirmed that BMSCs successfully differentiated into osteoblast with spread-out phenotype in HAp/collagen coated PCL scaffolds.
Conclusion: This study showed a proof of concept for preparing biomimetic 3D poly (ε-caprolactone)/ hydroxyapatite/collagen scaffolds with excellent osteoinduction and proliferation capacity for bone regeneration.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

