Impact of anesthesia and storage on posttranslational modifications of cardiac myofilament proteins
- PMID: 25952935
- PMCID: PMC4463824
- DOI: 10.14814/phy2.12393
Impact of anesthesia and storage on posttranslational modifications of cardiac myofilament proteins
Abstract
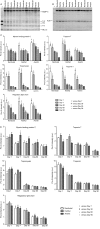
Although high fidelity measurements of posttranslational modifications (PTMs) of cardiac myofilament proteins exist, important issues remain regarding basic techniques of sample acquisition and storage. We investigated the effects of anesthetic regimen and sample storage conditions on PTMs of major ventricular sarcomeric proteins. Mice were anesthetized with pentobarbital (Nembutal), ketamine/xylazine mixture (Ket/Xyl), or tribromoethanol (Avertin), and the ventricular tissue was prepared and stored for 1, 7, 30, 60, or 90 days at -80°C. Myofilament protein phosphorylation and glutathionylation were analyzed by Pro-Q Diamond stain and Western blotting, respectively. With up to 7 days of storage, phosphorylation of troponin T was stable for samples from mice anesthetized with either Nembutal or Ket/Xyl but not Avertin; while myosin-binding protein C (MyBP-C) phosphorylation was reduced at 7 days with Nembutal and Ket/Xyl, though generally not significant until 90 days. Tropomyosin and regulatory myosin light chain phosphorylation were stable for up to 7 days regardless of the anesthetic regimen employed. In the case of Troponin I, by 7 days of storage there was a significant fall in phosphorylation across all anesthetics. Storage of samples from 30 to 90 days resulted in a general decrease in myofilament phosphorylation independent of the anesthetic. S-glutathionylation of MyBP-C presented a trend in reduced glutathionylation from days 30-90 in all anesthetics, with only day 90 being statistically significant. Our findings suggest that there are alterations in PTMs of major myofilament proteins from both storage and anesthetic selection, and that storage beyond 30 days will likely result in distortion of data.
Keywords: Anesthetic; cardiac myofilaments; posttranslational modifications; storage.
© 2015 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures



Similar articles
-
Comprehensive assessment of chamber-specific and transmural heterogeneity in myofilament protein phosphorylation by top-down mass spectrometry.J Mol Cell Cardiol. 2015 Oct;87:102-12. doi: 10.1016/j.yjmcc.2015.08.007. Epub 2015 Aug 9. J Mol Cell Cardiol. 2015. PMID: 26268593 Free PMC article.
-
Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice.Contemp Top Lab Anim Sci. 2005 Jan;44(1):17-22. Contemp Top Lab Anim Sci. 2005. PMID: 15697193
-
In vivo alpha-adrenergic responses and troponin I phosphorylation: anesthesia interactions.J Appl Physiol (1985). 2005 Apr;98(4):1163-70. doi: 10.1152/japplphysiol.00959.2004. Epub 2004 Dec 3. J Appl Physiol (1985). 2005. PMID: 15579573
-
Targeted proteomics of myofilament phosphorylation and other protein posttranslational modifications.Proteomics Clin Appl. 2014 Aug;8(7-8):543-53. doi: 10.1002/prca.201400034. Proteomics Clin Appl. 2014. PMID: 24976615 Review.
-
Posttranslational modifications of cardiac troponin T: an overview.J Mol Cell Cardiol. 2013 Oct;63:47-56. doi: 10.1016/j.yjmcc.2013.07.004. Epub 2013 Jul 18. J Mol Cell Cardiol. 2013. PMID: 23871791 Review.
Cited by
-
The Case for, and Challenges of, Human Cardiac Tissue in Advancing Phosphoprotein Research.Front Physiol. 2022 Mar 23;13:853511. doi: 10.3389/fphys.2022.853511. eCollection 2022. Front Physiol. 2022. PMID: 35399265 Free PMC article. Review.
-
Best practices in assessing cardiac protein O-GlcNAcylation by immunoblot.Am J Physiol Heart Circ Physiol. 2023 Oct 1;325(4):H601-H616. doi: 10.1152/ajpheart.00104.2023. Epub 2023 Aug 4. Am J Physiol Heart Circ Physiol. 2023. PMID: 37539459 Free PMC article.
-
Implications of S-glutathionylation of sarcomere proteins in cardiac disorders, therapies, and diagnosis.Front Cardiovasc Med. 2023 Jan 24;9:1060716. doi: 10.3389/fcvm.2022.1060716. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36762302 Free PMC article.
-
Protein Kinase D Plays a Crucial Role in Maintaining Cardiac Homeostasis by Regulating Post-Translational Modifications of Myofilament Proteins.Int J Mol Sci. 2024 Feb 28;25(5):2790. doi: 10.3390/ijms25052790. Int J Mol Sci. 2024. PMID: 38474037 Free PMC article.
-
O-GlcNAcylation levels remain stable regardless of the anaesthesia in healthy rats.Sci Rep. 2024 May 9;14(1):10669. doi: 10.1038/s41598-024-61445-0. Sci Rep. 2024. PMID: 38724577 Free PMC article.
References
-
- Davies DF. Effects of freezing and thawing serum and plasma on selected quantitative recoveries. Cryobiology. 1968;5:87–95. - PubMed
-
- Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B. Blumel G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate) Res Exp Med (Berl) 1984;184:159–169. - PubMed
-
- Farley AR. Link AJ. Identification and quantification of protein posttranslational modifications. Methods Enzymol. 2009;463:725–763. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

